Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10012)
| Target Name | Hydroperoxide isomerase ALOXE3 (ALOXE3) | ||||
|---|---|---|---|---|---|
| Synonyms |
Epidermis-type lipoxygenase 3; Hydroperoxy dehydratase ALOXE3; Hydroperoxy icosatetraenoate dehydratase; Hydroperoxy icosatetraenoate isomerase
Click to Show/Hide
|
||||
| Gene Name | ALOXE3 | ||||
| Sequence |
MAVYRLCVTTGPYLRAGTLDNISVTLVGTCGESPKQRLDRMGRDFAPGSVQKYKVRCTAE
LGELLLLRVHKERYAFFRKDSWYCSRICVTEPDGSVSHFPCYQWIEGYCTVELRPGTART ICQDSLPLLLDHRTRELRARQECYRWKIYAPGFPCMVDVNSFQEMESDKKFALTKTTTCV DQGDSSGNRYLPGFPMKIDIPSLMYMEPNVRYSATKTISLLFNAIPASLGMKLRGLLDRK GSWKKLDDMQNIFWCHKTFTTKYVTEHWCEDHFFGYQYLNGVNPVMLHCISSLPSKLPVT NDMVAPLLGQDTCLQTELERGNIFLADYWILAEAPTHCLNGRQQYVAAPLCLLWLSPQGA LVPLAIQLSQTPGPDSPIFLPTDSEWDWLLAKTWVRNSEFLVHENNTHFLCTHLLCEAFA MATLRQLPLCHPIYKLLLPHTRYTLQVNTIARATLLNPEGLVDQVTSIGRQGLIYLMSTG LAHFTYTNFCLPDSLRARGVLAIPNYHYRDDGLKIWAAIESFVSEIVGYYYPSDASVQQD SELQAWTGEIFAQAFLGRESSGFPSRLCTPGEMVKFLTAIIFNCSAQHAAVNSGQHDFGA WMPNAPSSMRQPPPQTKGTTTLKTYLDTLPEVNISCNNLLLFWLVSQEPKDQRPLGTYPD EHFTEEAPRRSIAAFQSRLAQISRDIQERNQGLALPYTYLDPPLIENSVSI Click to Show/Hide
|
||||
| Family | Lipoxygenase family | ||||
| Function |
Non-heme iron-containing lipoxygenase which is atypical in that it displays a prominent hydroperoxide isomerase activity and a reduced lipoxygenases activity. The hydroperoxide isomerase activity catalyzes the isomerization of hydroperoxides, derived from arachidonic and linoleic acid by ALOX12B, into hepoxilin-type epoxyalcohols and ketones. In presence of oxygen, oxygenates polyunsaturated fatty acids, including arachidonic acid, to produce fatty acid hydroperoxides. In the skin, acts downstream of ALOX12B on the linoleate moiety of esterified omega-hydroxyacyl-sphingosine (EOS) ceramides to produce an epoxy-ketone derivative, a crucial step in the conjugation of omega-hydroxyceramide to membrane proteins. Therefore plays a crucial role in the synthesis of corneocytes lipid envelope and the establishment of the skin barrier to water loss. In parallel, it may have a signaling function in barrier formation through the production of hepoxilins metabolites. Also plays a role in adipocyte differentiation through hepoxilin A3 and hepoxilin B3 production which in turn activate PPARG. Through the production of hepoxilins in the spinal cord, it may regulate inflammatory tactile allodynia.
Click to Show/Hide
|
||||
| Gene ID | 59344 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
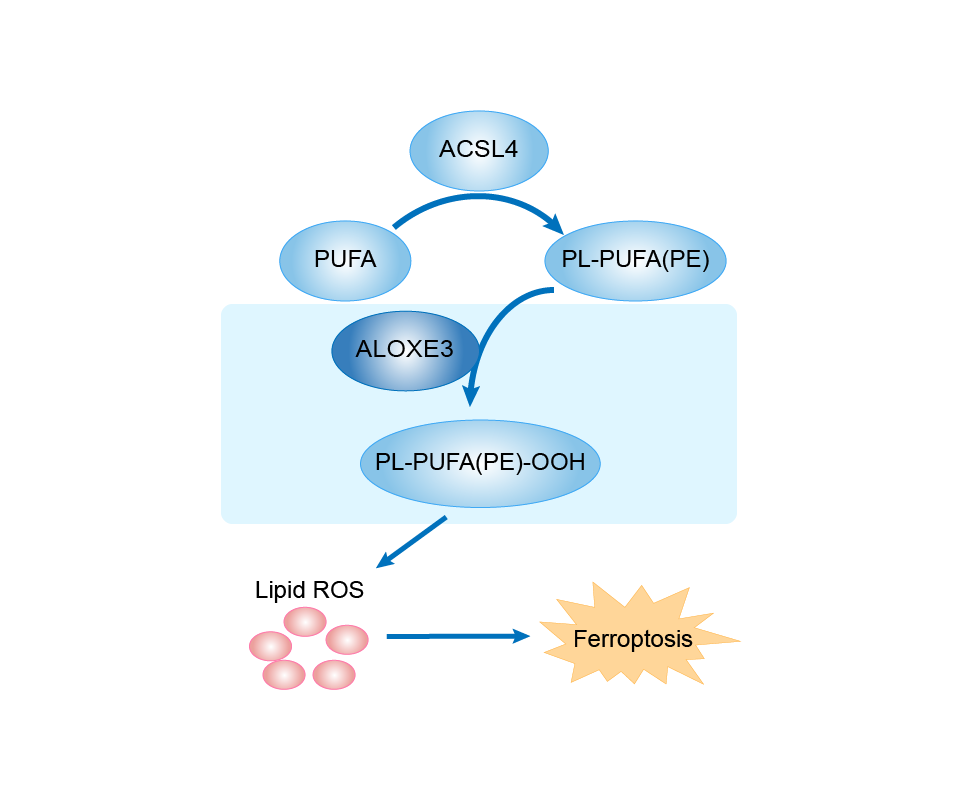
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
ALOXE3 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
hsa-mir-18a (Precursor RNA)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
The 8-week-old male nude mice were randomly divided into two groups (6 mice per group). The investigators were not blinded to the experimental groups. Orthotopic implantation of GBM cells into the hippocampus of nude mice was performed as we previously described (n = 6). Mice were intraperitoneally injected with luciferin (150 mg/kg; catalog #P1043; Promega) and subjected to IVIS Spectrum in vivo imaging system (PerkinElmer) to determine the intracranial tumor size.
Click to Show/Hide
|
||||
| Response Description | MiR-18a/ALOXE3 axis exerts tumor promoting functions by regulating ferroptosis and migration of glioblastoma cells. Mechanistically, miR-18a directly targeted ALOXE3 and suppressed its expression and functions in GBM cells. | ||||
Unspecific Regulator
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Responsed Drug | Talaroconvolutin A | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| In Vivo Model |
5 x 106 HCT116 cells were inoculated subcutaneously in the underarm of Balb/c nude female mice (5-week old). The inoculated mice were randomly divided into two groups (6 mice each group). When the tumor reached 300 mm3, the drug group was given TalaA intraperitoneally at a dose of 6.0 mg/kg, and the control group was given the same dose of cosolvent-corn oil. The drug (or cosolvent) was injected every 2 days. Body weight and tumor volume were measured every 2 days.
Click to Show/Hide
|
||||
| Response Description | Talaroconvolutin A (TalaA) downregulated the expression of the channel protein solute carrier family 7 member 11 (SLC7A11) but upregulated arachidonate lipoxygenase 3 (ALOXE3), promoting ferroptosis. TalaA causes upregulation of HMOX1 which lead to the degradation of heme and the release of free iron, accumulating in mitochondria and giving rise to lipid peroxidation. TalaA could be a new potential powerful drug candidate for colorectal cancer therapy. | ||||
Histone-lysine N-methyltransferase 2D (KMT2D)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell differentiation | |||||
In Vitro Model |
NHEK/SV3 cells | Normal | Homo sapiens | CVCL_9Q50 | |
| 3T3-J2 cells | Normal | Mus musculus | CVCL_W667 | ||
| In Vivo Model |
All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Mice were maintained on a mixed C57BL/6 background on a standard light-dark cycle. Mice carrying Mll4SET floxed alleles, Mll3SET floxed alleles, or a combination of both of these were crossed with Krt14-Cre transgenic mice.
Click to Show/Hide
|
||||
| Response Description | MLL4 (KMT2D) deficiency profoundly alters epidermal gene expression and uniquely rewires the expression of key genes and markers of ferroptosis (Alox12, Alox12b, and Aloxe3). | ||||
Unspecific Regulator
Talaroconvolutin A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| In Vivo Model |
5 x 106 HCT116 cells were inoculated subcutaneously in the underarm of Balb/c nude female mice (5-week old). The inoculated mice were randomly divided into two groups (6 mice each group). When the tumor reached 300 mm3, the drug group was given TalaA intraperitoneally at a dose of 6.0 mg/kg, and the control group was given the same dose of cosolvent-corn oil. The drug (or cosolvent) was injected every 2 days. Body weight and tumor volume were measured every 2 days.
Click to Show/Hide
|
||||
| Response Description | Talaroconvolutin A (TalaA) downregulated the expression of the channel protein solute carrier family 7 member 11 (SLC7A11) but upregulated arachidonate lipoxygenase 3 (ALOXE3), promoting ferroptosis. TalaA causes upregulation of HMOX1 which lead to the degradation of heme and the release of free iron, accumulating in mitochondria and giving rise to lipid peroxidation. TalaA could be a new potential powerful drug candidate for colorectal cancer therapy. | ||||
References
