Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0360)
| Name |
LCZ696
|
||||
|---|---|---|---|---|---|
| Synonyms |
LCZ-696; LCZ696 (Valsarta + sacubitril); sodium (S)-5-(4'-((N-(1-carboxylato-2-methylpropyl)pentanamido)methyl)-[1,1'-biphenyl]-2-yl)tetrazol-1-ide 4-(((2S,4R)-1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5-oxopentan-2-yl)amino)-4-oxobutanoate hydrate; LCZ-696A; BCP10630; Q6457467; Trisodium;4-[[(2S,4R)-5-ethoxy-4-methyl-5-oxo-1-(4-phenylphenyl)pentan-2-yl]amino]-4-oxobutanoate;(2S)-3-methyl-2-[pentanoyl-[[4-[2-(1,2,3-triaza-4-azanidacyclopenta-2,5-dien-5-yl)phenyl]phenyl]methyl]amino]butanoate;hydrate
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
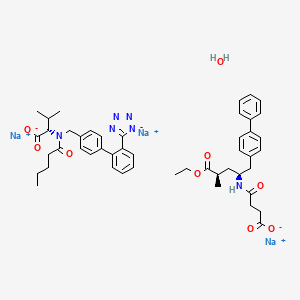 |
||||
|
3D MOL
|
|||||
| Formula |
C48H57N6Na3O9
|
||||
| IUPAC Name |
trisodium;4-[[(2S,4R)-5-ethoxy-4-methyl-5-oxo-1-(4-phenylphenyl)pentan-2-yl]amino]-4-oxobutanoate;(2S)-3-methyl-2-[pentanoyl-[[4-[2-(1,2,3-triaza-4-azanidacyclopenta-2,5-dien-5-yl)phenyl]phenyl]methyl]amino]butanoate;hydrate
|
||||
| Canonical SMILES |
CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NN=N[N-]3)C(C(C)C)C(=O)[O-].CCOC(=O)C(C)CC(CC1=CC=C(C=C1)C2=CC=CC=C2)NC(=O)CCC(=O)[O-].O.[Na+].[Na+].[Na+]
|
||||
| InChI |
InChI=1S/C24H29N5O3.C24H29NO5.3Na.H2O/c1-4-5-10-21(30)29(22(16(2)3)24(31)32)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-27-28-26-23;1-3-30-24(29)17(2)15-21(25-22(26)13-14-23(27)28)16-18-9-11-20(12-10-18)19-7-5-4-6-8-19;;;;/h6-9,11-14,16,22H,4-5,10,15H2,1-3H3,(H2,25,26,27,28,31,32);4-12,17,21H,3,13-16H2,1-2H3,(H,25,26)(H,27,28);;;;1H2/q;;3*+1;/p-3/t22-;17-,21+;;;;/m01..../s1
|
||||
| InChIKey |
UOLUPHRXIRFONO-JOYYXRJNSA-K
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-3, mitochondrial (SIRT3) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal protocols were approved by the Animal Care and Use Committee of TaizhouHospital, affiliated to Zhejiang University (Taizhou, China). Twenty-four 2-month-old male Wistar rats weighing 190-220 g were purchased from the Experimental Animal Center of Basi Medicine, Zhejiang Chinese Medical University. The animals were reared under a 12 h light/12 h dark cycle at a relative humidity of 55 ± 5% and temperature of 23 ± 2 , with unrestricted access to food and water. All animals were acclimatized to laboratory conditions for 1 week before the experiments and were randomly divided into four groups: control group (CG, n = 6); LCZ696 group (LCZ, n = 6); DOX group (DOX, n = 6); and DOX + LCZ696 group (DOX + LCZ, n = 6). The CG received saline solution by gavage for 6 weeks (2 mL/day), while the treatment groups received DOX (Cat. HY-15142A, MedChemExpress, USA), LCZ696 (Cat. HY-18204A, MedChemExpress, USA), or DOX + LCZ696. DOX was administered at a dose of 2.5 mg/kg once a week for 6 weeks via tailvein injection. LCZ696 (60 mg/kg/day) was administered by gavage for 6 weeks. Body weight was measured weekly.
Click to Show/Hide
|
||||
| Response regulation | LCZ696 treatment increased SIRT3 expression and deacetylated its target gene SOD2, and these changes were mediated by AKT activation. Collectively,LCZ696 prevents DOX-induced cardiotoxicity by inhibiting ferroptosis via AKT/SIRT3/SOD2 signaling pathway activation. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | Superoxide dismutase [Mn], mitochondrial (SOD2) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal protocols were approved by the Animal Care and Use Committee of TaizhouHospital, affiliated to Zhejiang University (Taizhou, China). Twenty-four 2-month-old male Wistar rats weighing 190-220 g were purchased from the Experimental Animal Center of Basi Medicine, Zhejiang Chinese Medical University. The animals were reared under a 12 h light/12 h dark cycle at a relative humidity of 55 ± 5% and temperature of 23 ± 2 , with unrestricted access to food and water. All animals were acclimatized to laboratory conditions for 1 week before the experiments and were randomly divided into four groups: control group (CG, n = 6); LCZ696 group (LCZ, n = 6); DOX group (DOX, n = 6); and DOX + LCZ696 group (DOX + LCZ, n = 6). The CG received saline solution by gavage for 6 weeks (2 mL/day), while the treatment groups received DOX (Cat. HY-15142A, MedChemExpress, USA), LCZ696 (Cat. HY-18204A, MedChemExpress, USA), or DOX + LCZ696. DOX was administered at a dose of 2.5 mg/kg once a week for 6 weeks via tailvein injection. LCZ696 (60 mg/kg/day) was administered by gavage for 6 weeks. Body weight was measured weekly.
Click to Show/Hide
|
||||
| Response regulation | LCZ696 treatment increased SIRT3 expression and deacetylated its target gene SOD2, and these changes were mediated by AKT activation. Collectively, LCZ696 prevents DOX-induced cardiotoxicity by inhibiting ferroptosis via AKT/SIRT3/SOD2 signaling pathway activation. | ||||
