Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0222)
| Name |
Lorlatinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Lorlatinib; 1454846-35-5; PF-06463922; Loratinib; Lorbrena; Lorviqua; PF06463922; PF 06463922; OSP71S83EU; PF06463922(Lorlatinib); CHEMBL3286830; PF-6463922; (10R)-7-amino-12-fluoro-10,15,16,17-tetrahydro-2,10,16-trimethyl-15-oxo-2H-4,8-methenopyrazolo[4,3-h][2,5,11]benzoxadiazacyclotetradecine-3-carbonitrile; (16R)-19-amino-13-fluoro-4,8,16-trimethyl-9-oxo-17-oxa-4,5,8,20-tetrazatetracyclo[16.3.1.02,6.010,15]docosa-1(22),2,5,10(15),11,13,18,20-octaene-3-carbonitrile; (R)-26-Amino-55-fluoro-11,4,7-trimethyl-6-oxo-11H-3-oxa-7-aza-2(3,5)-pyridina-1(4,3)-pyrazola-5(1,2)-benzenacyclooctaphane-15-carbonitrile; 2H-4,8-Methenopyrazolo(4,3-H)(2,5,11)benzoxadiazacyclotetradecine-3-carbonitrile, 7-amino-12-fluoro-10,15,16,17-tetrahydro-2,10,16-trimethyl-15-oxo-, (10R)-; (10r)-7-Amino-12-Fluoro-2,10,16-Trimethyl-15-Oxo-10,15,16,17-Tetrahydro-2h-8,4-(Metheno)pyrazolo[4,3-H][2,5,11]benzoxadiazacyclotetradecine-3-Carbonitrile; UNII-OSP71S83EU; lorlatinibum; Lorlatinib (PF-06463922); C21H19FN6O2; 4cli; 4clj; Lorbrena (TN); 2H-4,8-Methenopyrazolo[4,3-h][2,5,11]benzoxadiazacyclotetradecine-3-carbonitrile, 7-amino-12-fluoro-10,15,16,17-tetrahydro-2,10,16-trimethyl-15-oxo-, (10R)-; LORLATINIB [MI]; LORLATINIB [INN]; LORLATINIB [JAN]; Lorlatinib [USAN:INN]; LORLATINIB [USAN]; PFE-PKIS 10; LORLATINIB [WHO-DD]; 7-Amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo(4,3-h)(2,5,11)benzoxadiazacyclotetradecine-3-carbonitrile; Lorlatinib (JAN/USAN/INN); GTPL7476; LORLATINIB [ORANGE BOOK]; PF-06463922 Lorlatinib; SCHEMBL15261807; AMY3295; EX-A828; CHEBI:143117; DTXSID201027944; BCP10287; BDBM50018830; MFCD28144520; NSC780108; NSC800990; s7536; AKOS027250753; CCG-268718; CS-3983; DB12130; NSC-780108; NSC-800990; NCGC00386417-02; NCGC00386417-13; AC-30881; HY-12215; A14207; D11012; A857523; J-690185; Q27285820; (10R)-7-AMINO-12-FLUORO-2,10,16-TRIMETHYL-15-OXO-10,15,16,17-TETRAHYDRO-2H-4,8- METHENOPYRAZOLO(4,3-H)(2,5,11)BENZOXADIAZACYCLOTETRADECINE-3-CARBONITRILE; (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-4,8-methenopyrazolo[4,3-h][2,5,11]benzoxadiazacyclotetradecine-3-carbonitrile
Click to Show/Hide
|
||||
| Structure |
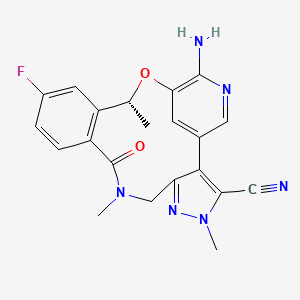 |
||||
| Formula |
C21H19FN6O2
|
||||
| IUPAC Name |
(16R)-19-amino-13-fluoro-4,8,16-trimethyl-9-oxo-17-oxa-4,5,8,20-tetrazatetracyclo[16.3.1.02,6.010,15]docosa-1(22),2,5,10(15),11,13,18,20-octaene-3-carbonitrile
|
||||
| Canonical SMILES |
CC1C2=C(C=CC(=C2)F)C(=O)N(CC3=NN(C(=C3C4=CC(=C(N=C4)N)O1)C#N)C)C
|
||||
| InChI |
InChI=1S/C21H19FN6O2/c1-11-15-7-13(22)4-5-14(15)21(29)27(2)10-16-19(17(8-23)28(3)26-16)12-6-18(30-11)20(24)25-9-12/h4-7,9,11H,10H2,1-3H3,(H2,24,25)/t11-/m1/s1
|
||||
| InChIKey |
IIXWYSCJSQVBQM-LLVKDONJSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Melanoma | ICD-11: 2C30 | |||
| Responsed Regulator | Insulin-like growth factor 1 receptor (IGF1R) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SK-MEL-28 cells | Cutaneous melanoma | Homo sapiens | CVCL_0526 | |
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | ||
| WM35 cells | Melanoma | Homo sapiens | CVCL_0580 | ||
| SK-MEL-5 cells | Cutaneous melanoma | Homo sapiens | CVCL_0527 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal experiments were approved by the Ethical Review of Experimental Animals at Central South University. To generate subcutaneous tumors, 2 x 106 control A375 cells or GPX4 KO cells were suspended in 100 ul PBS and injected subcutaneously into nude mice (Shanghai SLAC). Tumor-bearing mice were randomly allocated into groups and treated with vehicle (2% DMSO + 30% PEG300, per day by orally) or lorlatinib (10 mg/kg, per day by orally). Liproxstatin-1 (10 mg/kg) was administrated through intraperitoneal injection per day. Tumors were weighted and photographed on day 18 after treatment. Tumor size were recorded every three days and calculated as [(length x width x width)/2].
Click to Show/Hide
|
||||
| Response regulation | Lorlatinib sensitized melanoma to ferroptosis through targeting IGF1R-mediated PI3K/AKT/mTOR signaling axis and its downstream SCD expression. | ||||
Stearoyl-CoA desaturase (SCD)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Melanoma | ICD-11: 2C30 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SK-MEL-28 cells | Cutaneous melanoma | Homo sapiens | CVCL_0526 | |
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | ||
| WM35 cells | Melanoma | Homo sapiens | CVCL_0580 | ||
| SK-MEL-5 cells | Cutaneous melanoma | Homo sapiens | CVCL_0527 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal experiments were approved by the Ethical Review of Experimental Animals at Central South University. To generate subcutaneous tumors, 2 x 106 control A375 cells or GPX4 KO cells were suspended in 100 ul PBS and injected subcutaneously into nude mice (Shanghai SLAC). Tumor-bearing mice were randomly allocated into groups and treated with vehicle (2% DMSO + 30% PEG300, per day by orally) or lorlatinib (10 mg/kg, per day by orally). Liproxstatin-1 (10 mg/kg) was administrated through intraperitoneal injection per day. Tumors were weighted and photographed on day 18 after treatment. Tumor size were recorded every three days and calculated as [(length x width x width)/2].
Click to Show/Hide
|
||||
| Response regulation | Lorlatinib sensitized melanoma to ferroptosis through targeting IGF1R-mediated PI3K/AKT/mTOR signaling axis and its downstream SCD expression. | ||||
