Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0214)
| Name |
Kainic acid
|
||||
|---|---|---|---|---|---|
| Synonyms |
kainic acid; 487-79-6; Digenin; Digenic acid; Helminal; Kainate; L-alpha-Kainic acid; alpha-Kainic acid; Digensaeure; Kainsaeure; Acidum kainicum; Acide kainique; Acido kainico; C10H15NO4; 3-Pyrrolidineacetic acid, 2-carboxy-4-(1-methylethenyl)-, (2S,3S,4S)-; alpha- Kainic acid; (2S,3S,4S)-3-(Carboxymethyl)-4-(prop-1-en-2-yl)pyrrolidine-2-carboxylic acid; rac Kainic Acid; CHEMBL275040; SIV03811UC; DTXSID7040526; CHEBI:31746; 2-Carboxy-4-isopropenyl-3-pyrrolidineacetic acid; 3-Pyrrolidineacetic acid, 2-carboxy-4-isopropenyl-; NSC136038; NSC-759587; NCGC00024504-05; (3S,4R)-3-(carboxymethyl)-4-(prop-1-en-2-yl)-L-proline; 2-Carboxy-3-carboxymethyl-4-isopropenylpyrrolidine; NSC 136038; (2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid; DTXCID5020526; (3S,4S)-3-(carboxymethyl)-4-prop-1-en-2-yl-L-proline; (2S,3S,4S)-Carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid; Kainic acid [INN:JAN]; (2S,3S,4S)-3-(carboxymethyl)-4-prop-1-en-2-ylpyrrolidine-2-carboxylic acid; 3-(Carboxymethyl)-4-isopropenylproline; 4071-38-9; CAS-487-79-6; Acide kainique [INN-French]; Acido kainico [INN-Spanish]; Acidum kainicum [INN-Latin]; SR-01000075454; BRN 0086660; UNII-SIV03811UC; kainic-acid; NSC-136038; KAI; 1p1n; Kainic acid (synthetic); KAINIC ACID [MI]; Biomol-NT_000217; KAINIC ACID [INN]; UPCMLD-DP146; 3-Pyrrolidineacetic acid, 2-carboxy-4-(1-methylethenyl)-, (2S-(2alpha,3beta,4beta))-; Lopac0_000656; SCHEMBL15777; 4-22-00-01523 (Beilstein Handbook Reference); MLS001074661; KAINIC ACID [WHO-DD]; BPBio1_001306; (-)-(.alpha.)-Kainic Acid; UPCMLD-DP146:001; UPCMLD-DP146:002; HMS2233K05; HMS3262C13; HMS3266C11; HMS3411A21; HMS3675A21; HY-N2309; Tox21_110905; Tox21_500656; BDBM50002369; HB0355; Kainic Acid - CAS 487-79-6; AKOS024456995; Tox21_110905_1; CCG-204742; LP00656; SDCCGSBI-0050635.P002; 3-Pyrrolidineacetic acid, 2-carboxy-4-(1-methylethenyl)-, (2S-(2-alpha,3-beta,4-beta))-; NCGC00024504-02; NCGC00024504-03; NCGC00024504-04; NCGC00024504-06; NCGC00024504-07; NCGC00024504-08; NCGC00024504-09; NCGC00024504-15; NCGC00261341-01; SMR000471885; CS-0020451; EU-0100656; K 0250; Q390239; SR-01000597728; SR-01000075454-1; SR-01000075454-5; SR-01000075454-6; SR-01000075454-7; SR-01000597728-1; rel-(3R,4R)-3-(carboxymethyl)-4-isopropenyl-D-proline; 2S-CARBOXY-4S-(1-METHYLETHENYL)-3S-PYRROLDINEACETIC ACID; L-proline, 3-(carboxymethyl)-4-(1-methylethenyl)-, (3S,4S)-; (2S,3S,4S)-3-(Carboxymethyl)-4-Prop-1-En-2-yl-Pyrrolidine-2-Carboxylicacid; (2S-(2.ALPHA.,3.BETA.,4.BETA.))-2-CARBOXY-4-(1-METHYLETHENYL)-3-PYRROLIDINEACETIC ACID; InChI=1/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
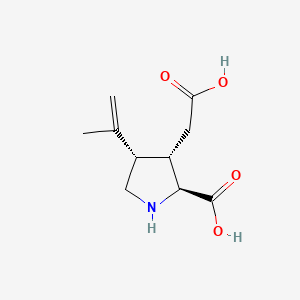 |
||||
| Formula |
C10H15NO4
|
||||
| IUPAC Name |
(2S,3S,4S)-3-(carboxymethyl)-4-prop-1-en-2-ylpyrrolidine-2-carboxylic acid
|
||||
| Canonical SMILES |
CC(=C)C1CNC(C1CC(=O)O)C(=O)O
|
||||
| InChI |
InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1
|
||||
| InChIKey |
VLSMHEGGTFMBBZ-OOZYFLPDSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
5-weeks-old kainate (KA)-induced BALB/c nude mice, a widely used epilepsy mouse model, were performed with intraperitoneal (i.p.) injection of KA (6 mg/kg). Pre-treatment 21 with antioxidant apigenin (60 mg/Kg, 2 days) or post-treatment with apigenin (60 mg/Kg, 1 day), mice were injected with KA (6 mg/kg) via intraperitoneal (i.p.) injection, and then HCP (0.5 mg/Kg) were injected by intravenous (i.v.) injection. In vivo and Ex vivo fluorescence images of relative ClO levels in mice brains 5, 15, 30, 45, and 60 min post injection of HCP were further performed by using the IVIS Spectrum imaging system (Nanjing University) with an excitation filter of 430 nm and the collection wavelength range is from 500-600 nm.
Click to Show/Hide
|
||||
| Response regulation | Apigenin can efficiently reduce the expression of intracellular MPO and increase the levels of GPX4 and SIRT1, thereby conferring neuroprotection through regulation of kainic acid (KA)-induced ferroptosis. And the level of Ac-p53 inside the brains treated with apigenin was down-regulated, suggesting that the p53-mediated ferroptosis pathway might be blocked. Overall, apigenin was screened and confirmed as an efficient lead compound for epilepsy prevention and treatment. | ||||
