Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0145)
| Name |
Ropivacaine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Ropivacaine; 84057-95-4; (S)-Ropivacaine; Naropin; Ropivacaine [INN]; Ropivacaine hydrochloride; Ropivacaina; Ropivacainum; Ropivacaina [Spanish]; S-Ropivacaine; (S)-N-(2,6-Dimethylphenyl)-1-propylpiperidine-2-carboxamide; Ropivacainum [INN-Latin]; Ropivacaina [INN-Spanish]; (2S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide; Ropivicaine; Ropivacaine base; (2S)-N-(2,6-dimethylphenyl)-1-propyl-piperidine-2-carboxamide; 7IO5LYA57N; (-)-1-Propyl-2',6'-pipecoloxylidide; LEA 103; CHEBI:8890; TLC590; (2S)-N-(2,6-dimethylphenyl)-1-propyl-2-piperidinecarboxamide; TLC-590; Ropivacaine (INN); MFCD00864425; L-N-n-propylpipecolic acid-2,6-xylidide; 2-Piperidinecarboxamide, N-(2,6-dimethylphenyl)-1-propyl-, (2S)-; (S)-(-)-1-propyl-2',6'-pipecoloxylidide; 1-propyl-2',6'-pipecoloxylidide; Noropine; Narop; LEA-103 HCl; Naropin (TN); LEA-103; NCGC00164597-01; Ropivacaine [INN:BAN]; UNII-7IO5LYA57N; AL-381; ROPIVACAINE [MI]; ROPIVACAINE [VANDF]; SCHEMBL33292; ROPIVACAINE [USP-RS]; ROPIVACAINE [WHO-DD]; BIDD:GT0203; Narop; Noropine; LEA-103; GTPL7602; (-)-1-Propyl-2',6'-dimethyl-2-piperidylcarboxyanilid; CHEMBL1077896; DTXSID4040187; ZKMNUMMKYBVTFN-HNNXBMFYSA-N; HY-B0563; BBL102321; s5504; STL556120; AKOS017343283; CCG-267197; DB00296; AS-35173; CS-0009514; R0251; C07532; D08490; AB00698466-07; AB00698466_10; EN300-7436767; A840710; Q279504; (S)-(-)-1-PROPYL-2',6'-PIPECOLOXYLIDINE; Q-201677; (-)-1-propyl-2',6'-dimethyl-2-piperidylcarboxyanilide; Z2235811359; (s)-n-(2,6-dimethylphenyl)-1-propyl-2-piperidinecarboxamide; (S)-(-)-1-PROPYLPIPERIDINE-2-CARBOXYLIC ACID (2,6-DIMETHYLPHENYL)AMIDE
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
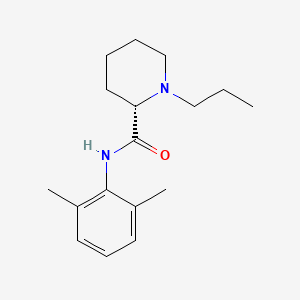 |
||||
| Formula |
C17H26N2O
|
||||
| IUPAC Name |
(2S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide
|
||||
| Canonical SMILES |
CCCN1CCCCC1C(=O)NC2=C(C=CC=C2C)C
|
||||
| InChI |
InChI=1S/C17H26N2O/c1-4-11-19-12-6-5-10-15(19)17(20)18-16-13(2)8-7-9-14(16)3/h7-9,15H,4-6,10-12H2,1-3H3,(H,18,20)/t15-/m0/s1
|
||||
| InChIKey |
ZKMNUMMKYBVTFN-HNNXBMFYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Ovarian cancer | ICD-11: 2C73 | |||
| Responsed Regulator | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) | Suppressor | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Twelve Nude female BALB/c-nu mice (5-weeks-old) were from Shanghai Lab. Animal Research Center (Shanghai, China). SKOV3 cells (5 x 106) were injected subcutaneously into mice according to the previously described methods with minor changes. To evaluate the effect of ropivacaine on the growth of ovarian cancer, ropivacaine (10 mg/kg) was injected intraperitoneally into mice referring to the previously reported methods with minor revisions. The size of the tumor was measured every day and the tumor volumes were calculated by the formula: length x width2/2 = tumor volume (mm3). When the tumor size reached 2000 mm3, all mice were sacrificed and the excised tumor tissues were weighed to evaluate the antitumor effect.
Click to Show/Hide
|
||||
| Response regulation | The mechanism results confirmed that ropivacaine inactivated the PI3K/AKT signaling pathway in ovarian cancer cells. Furthermore, in vivo assay demonstrated that ropivacaine repressed the proliferation of ovarian cancer cells in vivo and had a protective function in ovarian cancer. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Ovarian cancer | ICD-11: 2C73 | |||
| Responsed Regulator | RAC-alpha serine/threonine-protein kinase (AKT1) | Suppressor | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Twelve Nude female BALB/c-nu mice (5-weeks-old) were from Shanghai Lab. Animal Research Center (Shanghai, China). SKOV3 cells (5 x 106) were injected subcutaneously into mice according to the previously described methods with minor changes. To evaluate the effect of ropivacaine on the growth of ovarian cancer, ropivacaine (10 mg/kg) was injected intraperitoneally into mice referring to the previously reported methods with minor revisions. The size of the tumor was measured every day and the tumor volumes were calculated by the formula: length x width2/2 = tumor volume (mm3). When the tumor size reached 2000 mm3, all mice were sacrificed and the excised tumor tissues were weighed to evaluate the antitumor effect.
Click to Show/Hide
|
||||
| Response regulation | The mechanism results confirmed that ropivacaine inactivated the PI3K/AKT signaling pathway in ovarian cancer cells. Furthermore, in vivo assay demonstrated that ropivacaine repressed the proliferation of ovarian cancer cells in vivo and had a protective function in ovarian cancer. | ||||
