Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0077)
| Name |
Temozolomide
|
||||
|---|---|---|---|---|---|
| Synonyms |
temozolomide; 85622-93-1; Methazolastone; Temodar; Temodal; Temozolamide; 3-methyl-4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide; Sch 52365; CCRG-81045; Temozolomidum [Latin]; Temozolodida [Spanish]; Temozolomidum; CCRG 81045; NSC 362856; Sch-52365; M&B 39831; M&B-39831; NSC-362856; C6H6N6O2; CCRIS 8996; MB 39831; 8-Carbamoyl-3-methylimidazo(5,1-d)-1,2,3,5-tetrazin-4(3H)-one; M & B 39831; BRN 5547136; M-39831; 3,4-Dihydro-3-methyl-4-oxoimidazo(5,1-d)-1,2,3,5-tetrazine-8-carboxamide; 3-Methyl-4-oxo-3,4-dihydroimidazo(5,1-d)(1,2,3,5)tetrazine-8-carboxamide; NSC362856; CHEMBL810; 3,4-Dihydro-3-methyl-4-oxoimidazo(5,1-d)-as-tetrazine-8-carboxamide; MLS002701861; YF1K15M17Y; DTXSID5043714; CHEBI:72564; 3-methyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide; Mk-7365; 3-methyl-4-oxo-3H,4H-imidazo[4,3-d][1,2,3,5]tetrazine-8-carboxamide; TMZ; NCGC00167429-01; Temozolodida; 8-CARBAMOYL-3-METHYLIMIDAZO[5,1-D]-1,2,3,5-TETRAZIN-4(3H)-ONE; DTXCID3023714; 3,4-dihydro-3-methyl-4-oxoimidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide; 3-methyl-4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide.; 3-Methyl-4-oxo-3,4-dihydro-imidazo[5,1-d][1,2,3,5]tetrazine-8-carboxylic acid amide; SMR000466338; Temodal (TN); Temodar (TN); CAS-85622-93-1; Temozolomide, VETRANAL(TM), analytical standard; SR-01000759347; temozolomida; UNII-YF1K15M17Y; Temozolomide (JAN/USAN/INN); Temozolomide [USAN:INN:BAN]; 3,4-Dihydro-3-methyl-4-oxoimidazo[5,1-d]-as-tetrazine-8-carboxamide; MFCD00866492; Temozolomide- Bio-X; TEMOZOLOMIDE [MI]; TEMOZOLOMIDE [INN]; TEMOZOLOMIDE [JAN]; Temodar (TN) (Schering); TEMOZOLOMIDE [USAN]; SCHEMBL3739; 4-methyl-5-oxo-2,3,4,6,8-pentazabicyclo[4.3.0]nona-2,7,9-triene-9-carboxamide; TEMOZOLOMIDE [VANDF]; TEMOZOLOMIDE [MART.]; MLS000759447; MLS001424028; BIDD:GT0204; TEMOZOLOMIDE [USP-RS]; TEMOZOLOMIDE [WHO-DD]; GTPL7301; TEMOZOLOMIDE [EMA EPAR]; Temozolomide, >=98% (HPLC); BPEGJWRSRHCHSN-UHFFFAOYSA-N; HMS2051O12; HMS2090B08; HMS2232N13; HMS3264I14; HMS3269P05; HMS3372K13; HMS3393O12; HMS3413D06; HMS3654N05; HMS3677D06; HMS3713H16; Pharmakon1600-01502289; TEMOZOLOMIDE [ORANGE BOOK]; TEMOZOLOMIDE [EP MONOGRAPH]; 3-methyl-4-oxo-imidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide; BCP03692; TEMOZOLOMIDE [USP MONOGRAPH]; Tox21_112433; AC-758; BDBM50034562; DL-190; NSC759883; s1237; STK623541; 3,4-Dihydro-3-methyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide; 3-Methyl-4-oxo-3,4-dihydroimidazo-[5,1-d][1,2,3,5]tetrazine-8-carboxamide; 3-Methyl-4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetraazine-8-carboxamide; AKOS005557098; Tox21_112433_1; CCG-100870; CS-0943; DB00853; KS-1216; NC00120; NSC-759883; Imidazo(5,1-d)(1,2,3,5)tetrazine-8-carboxamide, 3,4-dihydro-3-methyl-4-oxo-; NCGC00167429-02; NCGC00167429-04; NCGC00167429-05; BP-25388; BT164447; HY-17364; NCI60_003316; BCP0726000154; Temozolomide 100 microg/mL in Acetonitrile; AM20110227; FT-0630936; FT-0674845; SW197500-4; T2744; D06067; EN300-122324; AB00639915-06; AB00639915-08; AB00639915-09; AB00639915_10; AB00639915_11; A841386; Q425088; Q-201786; SR-01000759347-4; SR-01000759347-5; BRD-K32107296-001-04-5; Z1201620684; 3-methyl-4-oxo-8-imidazo[5,1-d][1,2,3,5]tetrazinecarboxamide; Temozolomide, United States Pharmacopeia (USP) Reference Standard; 3-methyl-4-oxidanylidene-imidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide; 3-Methyl-8-aminocarbonyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one; Imidazo[5,2,3,5-tetrazine-8-carboxamide, 3,4-dihydro-3-methyl-4-oxo-; {Imidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide,} 3, 4-dihydro-3-methyl-4-oxo-; Temozolomide, Pharmaceutical Secondary Standard; Certified Reference Material; 3-Methyl-4-oxo-3,4-dihydro-imidazo[5,1-d][1,2,3,5]tetrazine-8-carboxylic acid amide (Temozolomide); 3-Methyl-4-oxo-3,4-dihydro-imidazo[5,1-d][1,2,3,5]tetrazine-8-carboxylic acid amide(Temozolomide)
Click to Show/Hide
|
||||
| Structure |
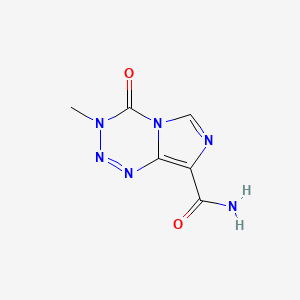 |
||||
| Formula |
C6H6N6O2
|
||||
| IUPAC Name |
3-methyl-4-oxoimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide
|
||||
| Canonical SMILES |
CN1C(=O)N2C=NC(=C2N=N1)C(=O)N
|
||||
| InChI |
InChI=1S/C6H6N6O2/c1-11-6(14)12-2-8-3(4(7)13)5(12)9-10-11/h2H,1H3,(H2,7,13)
|
||||
| InChIKey |
BPEGJWRSRHCHSN-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Natural resistance-associated macrophage protein 2 (SLC11A2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Driver | |||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | TG905 (Human glioblastoma cells) | |||
| Response regulation | Temozolomide may suppress cell growth partly by inducing ferroptosis by targeting DMT1 expression in glioblastoma cells. The results also showed that temozolomide-induced ferroptosis is associated with regulation of the Nrf2/HO-1 pathway. | |||
