Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10019)
| Target Name | CDGSH iron-sulfur domain-containing protein 2 (CISD2) | ||||
|---|---|---|---|---|---|
| Synonyms |
Endoplasmic reticulum intermembrane small protein; MitoNEET-related 1 protein; Nutrient-deprivation autophagy factor-1
Click to Show/Hide
|
||||
| Gene Name | CISD2 | ||||
| Sequence |
MVLESVARIVKVQLPAYLKRLPVPESITGFARLTVSEWLRLLPFLGVLALLGYLAVRPFL
PKKKQQKDSLINLKIQKENPKVVNEINIEDLCLTKAAYCRCWRSKTFPACDGSHNKHNEL TGDNVGPLILKKKEV Click to Show/Hide
|
||||
| Family | CISD protein family | ||||
| Function |
Regulator of autophagy that contributes to antagonize BECN1- mediated cellular autophagy at the endoplasmic reticulum. Participates in the interaction of BCL2 with BECN1 and is required for BCL2-mediated depression of endoplasmic reticulum Ca(2+) stores during autophagy. Contributes to BIK-initiated autophagy, while it is not involved in BIK-dependent activation of caspases. Involved in life span control, probably via its function as regulator of autophagy.
Click to Show/Hide
|
||||
| Gene ID | 493856 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
|
|||||
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
CISD2 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Unspecific Regulator
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 |
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | |
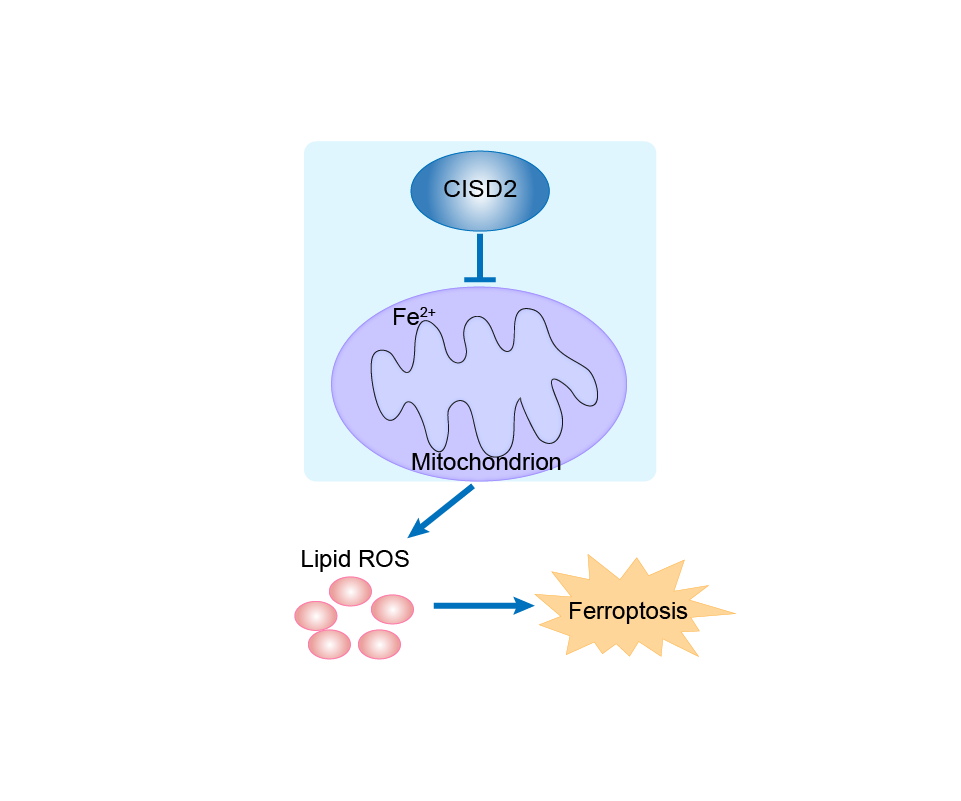
| Response Description | Inhibition of CISD2 promoted sorafenib-induced ferroptosis in resistant cells, and this process promoted excessive iron ion accumulation through autophagy, leading to ferroptosis. The combination of CISD2 inhibition and sorafenib treatment is an effective therapeutic strategy for resistant hepatocellular carcinoma (HCC). | |||
Head neck squamous cell carcinoma [ICD-11: 2D60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
AMC-HN-2 cells | Hypopharyngeal squamous cell carcinoma | Homo sapiens | CVCL_5960 | |
| SNU-1041 cells | Hypopharyngeal squamous cell carcinoma | Homo sapiens | CVCL_L085 | ||
| SNU-1066 cells | Laryngeal squamous cell carcinoma | Homo sapiens | CVCL_5005 | ||
| SNU-1076 cells | Laryngeal squamous cell carcinoma | Homo sapiens | CVCL_5006 | ||
| In Vivo Model |
Six-week-old athymic BALB/c malenude mice(nu/nu) were purchased from the Central Lab Animal Inc. (Seoul, Republic of Korea). SAS-resistant HN10 cells were injected subcutaneously into the flank of nude mice. Following the detection of gross nodules from the tumor implants, the mice were subjected to four different treatments: vehicle, SAS (250mg/kg daily per intraperitoneal route), PGZ (20 mg/kg daily per oral administration), or SAS plus PGZ.
Click to Show/Hide
|
||||
| Response Description | CISD2 inhibition overcomes head and neck cancer (HNC) resistance to ferroptotic cell death induced by sulfasalazine via increased accumulation of mitochondrial ferrous iron and lipid ROS. | ||||
References
