Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10004)
| Target Name | Long-chain-fatty-acid--CoA ligase 1 (ACSL1) | ||||
|---|---|---|---|---|---|
| Synonyms |
Acyl-CoA synthetase 1; Arachidonate--CoA ligase; Long-chain acyl-CoA synthetase 1; Long-chain acyl-CoA synthetase 2; Long-chain fatty acid-CoA ligase 2; Palmitoyl-CoA ligase 1; Palmitoyl-CoA ligase 2; Phytanate--CoA ligase
Click to Show/Hide
|
||||
| Gene Name | ACSL1 | ||||
| Sequence |
MQAHELFRYFRMPELVDFRQYVRTLPTNTLMGFGAFAALTTFWYATRPKPLKPPCDLSMQ
SVEVAGSGGARRSALLDSDEPLVYFYDDVTTLYEGFQRGIQVSNNGPCLGSRKPDQPYEW LSYKQVAELSECIGSALIQKGFKTAPDQFIGIFAQNRPEWVIIEQGCFAYSMVIVPLYDT LGNEAITYIVNKAELSLVFVDKPEKAKLLLEGVENKLIPGLKIIVVMDAYGSELVERGQR CGVEVTSMKAMEDLGRANRRKPKPPAPEDLAVICFTSGTTGNPKGAMVTHRNIVSDCSAF VKATENTVNPCPDDTLISFLPLAHMFERVVECVMLCHGAKIGFFQGDIRLLMDDLKVLQP TVFPVVPRLLNRMFDRIFGQANTTLKRWLLDFASKRKEAELRSGIIRNNSLWDRLIFHKV QSSLGGRVRLMVTGAAPVSATVLTFLRAALGCQFYEGYGQTECTAGCCLTMPGDWTAGHV GAPMPCNLIKLVDVEEMNYMAAEGEGEVCVKGPNVFQGYLKDPAKTAEALDKDGWLHTGD IGKWLPNGTLKIIDRKKHIFKLAQGEYIAPEKIENIYMRSEPVAQVFVHGESLQAFLIAI VVPDVETLCSWAQKRGFEGSFEELCRNKDVKKAILEDMVRLGKDSGLKPFEQVKGITLHP ELFSIDNGLLTPTMKAKRPELRNYFRSQIDDLYSTIKV Click to Show/Hide
|
||||
| Family | ATP-dependent AMP-binding enzyme family | ||||
| Function |
Catalyzes the conversion of long-chain fatty acids to their active form acyl-CoAs for both synthesis of cellular lipids, and degradation via beta-oxidation. Preferentially uses palmitoleate, oleate and linoleate. Preferentially activates arachidonate than epoxyeicosatrienoic acids (EETs) or hydroxyeicosatrienoic acids (HETEs).
Click to Show/Hide
|
||||
| Gene ID | 2180 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
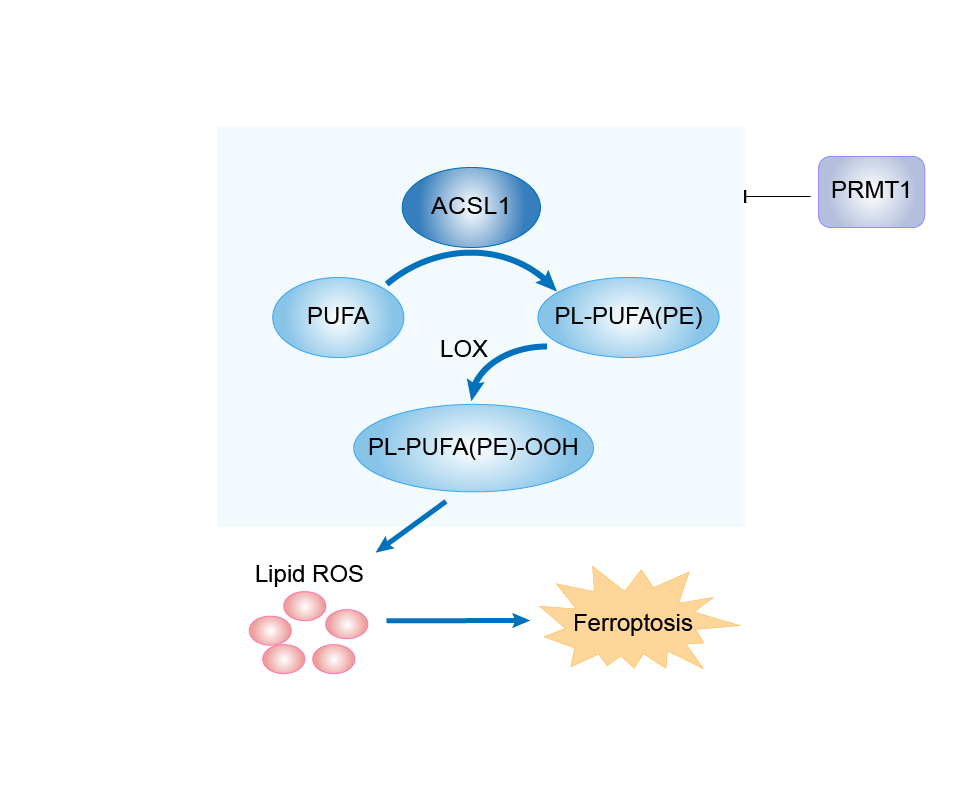
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
ACSL1 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Protein arginine N-methyltransferase 1 (PRMT1)
Acute myeloid leukaemia [ICD-11: 2A60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | GSK3368715 | Phase 1 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
NB4 cells | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | |
| HEL cells | Erythroleukemia | Homo sapiens | CVCL_0001 | ||
| MOLM-13 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_2119 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
5 x 106 NB4 cells were subcutaneously injected into the flank of 6-7-week-old female nude mice. The tumor number, body weight, and tumor volume were measured every other day. Tumor volumes were estimated using the following formula: tumor volume = (length x width2)/2. When tumor volumes reached 100-200 mm3, the mice were randomly divided into solvent control, RSL3, GSK3368715, or RSL3 + GSK3368715 combination groups (six mice per group). Specifically, for the RSL3 group, mice received intraperitoneal injections of RSL3 at 50 mg/kg 2 days apart. In the GSK3368715 group, GSK3368715 was intraperitoneally injected into mice at a dose of 75 mg/kg for 2 consecutive days per week. For the group that received RSL3 + GSK3368715 combination treatment, GSK3368715 (75 mg/kg) was administered by intraperitoneal injection for the first 2 days. Immediately thereafter, the mice received an intraperitoneal injection of RSL3 (50 mg/kg) 2 days apart. After 1 day of rest, the cycle was repeated until the end of the study on Day 21. Tumor volumes and body weights of the mice were measured and recorded every other day.
Click to Show/Hide
|
||||
| Response Description | Both GSK3368715 and PRMT1 knockout upregulated acyl-CoA synthetase long-chain family member 1 (ACSL1), which acts as a ferroptosis promoter by increasing lipid peroxidation. Knockout ACSL1 reduced the ferroptosis sensitivity of Acute myeloid leukemia (AML) cells following GSK3368715 treatment. Overall, PRMT1 inhibition promotes ferroptosis sensitivity via ACSL1 upregulation in acute myeloid leukemia. | ||||
Unspecific Regulator
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Responsed Drug | alpha-Eleostearic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MDA-MB-468 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| BT-20 cells | Invasive breast carcinoma of no special type | Homo sapiens | CVCL_0178 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| HCC38 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1267 | ||
| HCC1806 cells | Breast squamous cell carcinoma | Homo sapiens | CVCL_1258 | ||
| HCC1187 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1247 | ||
| HCC1143 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1245 | ||
| HCC70 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1270 | ||
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| MCF-12A cells | Normal | Homo sapiens | CVCL_3744 | ||
| In Vivo Model |
Mice were housed in a dedicated laboratory animal facility with 12-h light:dark cycle, at 70F+/-2 degrees, and 40-70% relative humidity. Orthotopic xenografts were generated by implanting 2.5 million MDA-MB-231 cells in 100 uL phosphate-buffered saline (PBS) mixed with 100 uL growth factor-reduced Matrigel (Corning) bilaterally into the fourth inguinal fat pad of four- to six-week-old female NOD.
Click to Show/Hide
|
||||
| Response Description | a-eleostearic acid (ESA)-triggered ferroptosis is mediated by acyl-CoA synthetase long-chain isoform 1 (ACSL1), which promotes ESA incorporation into neutral lipids including triacylglycerols in Breast adenocarcinoma. | ||||
Protein arginine N-methyltransferase 1 (PRMT1)
GSK3368715
[Phase 1]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Acute myeloid leukaemia [ICD-11: 2A60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | NB4 cells | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | |
| HEL cells | Erythroleukemia | Homo sapiens | CVCL_0001 | ||
| MOLM-13 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_2119 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
5 x 106 NB4 cells were subcutaneously injected into the flank of 6-7-week-old female nude mice. The tumor number, body weight, and tumor volume were measured every other day. Tumor volumes were estimated using the following formula: tumor volume = (length x width2)/2. When tumor volumes reached 100-200 mm3, the mice were randomly divided into solvent control, RSL3, GSK3368715, or RSL3 + GSK3368715 combination groups (six mice per group). Specifically, for the RSL3 group, mice received intraperitoneal injections of RSL3 at 50 mg/kg 2 days apart. In the GSK3368715 group, GSK3368715 was intraperitoneally injected into mice at a dose of 75 mg/kg for 2 consecutive days per week. For the group that received RSL3 + GSK3368715 combination treatment, GSK3368715 (75 mg/kg) was administered by intraperitoneal injection for the first 2 days. Immediately thereafter, the mice received an intraperitoneal injection of RSL3 (50 mg/kg) 2 days apart. After 1 day of rest, the cycle was repeated until the end of the study on Day 21. Tumor volumes and body weights of the mice were measured and recorded every other day.
Click to Show/Hide
|
||||
| Response Description | Both GSK3368715 and PRMT1 knockout upregulated acyl-CoA synthetase long-chain family member 1 (ACSL1), which acts as a ferroptosis promoter by increasing lipid peroxidation. Knockout ACSL1 reduced the ferroptosis sensitivity of Acute myeloid leukemia (AML) cells following GSK3368715 treatment. Overall, PRMT1 inhibition promotes ferroptosis sensitivity via ACSL1 upregulation in acute myeloid leukemia. | ||||
Unspecific Regulator
alpha-Eleostearic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MDA-MB-468 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| BT-20 cells | Invasive breast carcinoma of no special type | Homo sapiens | CVCL_0178 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| HCC38 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1267 | ||
| HCC1806 cells | Breast squamous cell carcinoma | Homo sapiens | CVCL_1258 | ||
| HCC1187 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1247 | ||
| HCC1143 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1245 | ||
| HCC70 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1270 | ||
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| MCF-12A cells | Normal | Homo sapiens | CVCL_3744 | ||
| In Vivo Model |
Mice were housed in a dedicated laboratory animal facility with 12-h light:dark cycle, at 70F+/-2 degrees, and 40-70% relative humidity. Orthotopic xenografts were generated by implanting 2.5 million MDA-MB-231 cells in 100 uL phosphate-buffered saline (PBS) mixed with 100 uL growth factor-reduced Matrigel (Corning) bilaterally into the fourth inguinal fat pad of four- to six-week-old female NOD.
Click to Show/Hide
|
||||
| Response Description | a-eleostearic acid (ESA)-triggered ferroptosis is mediated by acyl-CoA synthetase long-chain isoform 1 (ACSL1), which promotes ESA incorporation into neutral lipids including triacylglycerols in Breast adenocarcinoma. | ||||
References
