Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0354)
| Name |
SRS 16-86
|
||||
|---|---|---|---|---|---|
| Synonyms |
SRS16-86; 1793052-96-6; tert-butyl 4-(1-adamantylamino)-3-(pyrimidin-5-ylmethylideneamino)benzoate; 3-[(Z)-(5-pyrimidinylmethylene)amino]-4-(tricyclo[3.3.1.13,7]dec-1-ylamino)-benzoic acid, 1,1-dimethylethyl ester; CHEMBL3781699; SCHEMBL16799435; SCHEMBL16801340; SCHEMBL21394329; CHEBI:173096; s9840; SRS 16-86; AKOS040759650; MS-27720; HY-135430; CS-0112778; (Z)-tert-Butyl 4-(adamantan-1-ylamino)-3-((pyrimidin-5-ylmethylene)amino)benzoate; tert-butyl 4-[(adamantan-1-yl)amino]-3-{(Z)-[(pyrimidin-5-yl)methylidene]amino}benzoate; 3-[(Z)-(5-pyrimidinylmethylene)amino]-4-(tricyclo[3.3.1.1(3,7)]dec-1-ylamino)-benzoic acid 1,1-dimethylethyl ester; tert-butyl 3-[(Z)-(pyrimidin-5-ylmethylidene)amino]-4-(tricyclo[3.3.1.1(3,7)]decan-1-ylamino)benzoate
Click to Show/Hide
|
||||
| Structure |
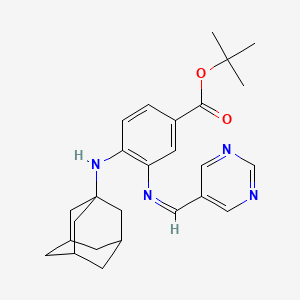 |
||||
| Formula |
C26H32N4O2
|
||||
| IUPAC Name |
tert-butyl 4-(1-adamantylamino)-3-(pyrimidin-5-ylmethylideneamino)benzoate
|
||||
| Canonical SMILES |
CC(C)(C)OC(=O)C1=CC(=C(C=C1)NC23CC4CC(C2)CC(C4)C3)N=CC5=CN=CN=C5
|
||||
| InChI |
InChI=1S/C26H32N4O2/c1-25(2,3)32-24(31)21-4-5-22(23(9-21)29-15-20-13-27-16-28-14-20)30-26-10-17-6-18(11-26)8-19(7-17)12-26/h4-5,9,13-19,30H,6-8,10-12H2,1-3H3
|
||||
| InChIKey |
DFENTOUMMDWZAF-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Spinal cord injury | ICD-11: ND51 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rSCs (Rat spinal cords) | ||||
| In Vivo Model |
Female Wistar rats (n = 120) weighing 240 ± 10 g were purchased from Laboratory Animal Center of the Academy of Military Medical Sciences (Beijing, China). To optimize the SRS 16-86 dose, 20 animals were divided into five groups to test the locomotor recovery at 2 weeks after SCI (n = 4 per group, Sham, SCI-vehicle, 5 mg/kg, 10 mg/kg and 15 mg/kg of SRS 16-86 intraperitoneally). According to the two week preliminary experiments, the dose of 15 mg/kg SRS 16-86 was used in the following experiments. For the TEM observation, 15 animals were divided into five groups (n = 3, Sham, SCI-15min, SCI-1 h, SCI 4 h and SCI 24 h to see the mitochondria changes. Another 9 animals were assigned to detect the TEM of spinal cord at 24 h post injury (n = 3 per group, Sham, SCI-vehicle and SCI-SRS 16-86). 18 animals were used (n = 6 per group, Sham, SCI-vehicle and SCI-SRS 16-86) to observe the hindlimb function at 1 d, 7 d, 14 d, 28 d, 42 d and 56 d after SCI.
Click to Show/Hide
|
||||
| Response regulation | SRS 16-86 treatment alleviated astrogliosis and enhanced neuronal survival after spinal cord injury (SCI). The inflammatory cytokine levels (IL-1, TNF- and ICAM-1) were decreased significantly post SRS 16-86 treatment after SCI. These findings suggest strong correlation between ferroptosis and the secondary injury of SCI. | ||||
