Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0336)
| Name |
4-Methylumbelliferyl beta-D-xylopyranoside
|
||||
|---|---|---|---|---|---|
| Synonyms |
6734-33-4; 4-Methylumbelliferyl beta-D-xylopyranoside; 4-Methylumbelliferyl-beta-D-xylopyranoside; 4-Methylumbelliferyl b-D-xylopyranoside; 4-Methylumbelliferyl beta-D-xyloside; Methylumbelliferyl-beta-D-xyloside; CHEMBL1938471; 4-Methylumbelliferyl beta-xyloside; 4-methyl-7-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxychromen-2-one; EINECS 229-784-4; MFCD00037607; 766N8850JA; 4-Methylumbelliferyl-; A-D-xylopyranoside; 4-Methyl-7-(beta-D-xylopyranosyloxy)-2H-1-benzopyran-2-one; 2H-1-Benzopyran-2-one, 4-methyl-7-(.beta.-D-xylopyranosyloxy)-; 4'-Methylumbelliferyl-b-D-xylose; 4-Methylumbelliferyl-beta-D-xyloside; 4-Methylumbelliferylb-D-xylopyranoside; SCHEMBL197177; UNII-766N8850JA; DTXSID10217687; 4-methylumbelliferyl-beta-xyloside; 2H-1-Benzopyran-2-one, 4-methyl-7-(b-D-xylopyranosyloxy)-; 2H-1-Benzopyran-2-one,4-methyl-7-(b-D-xylopyranosyloxy)-; 2H-1-Benzopyran-2-one, 4-methyl-7-(beta-D-xylopyranosyloxy)-; AMY41722; BDBM50361734; AKOS005257904; AKOS015899834; s12045; AS-70369; PD171160; 4-Methylumbelliferyl I(2)-D-xylopyranoside; HY-137824; 4-Methylumbelliferyl- beta -D-xylopyranoside; (4-Methylumbelliferone)-beta-D-xylopyranoside; Boc-(S)-3-Amino-4-(2-thienyl)butanoicacid; 4-METHYLUMBELLIFERONE-.BETA.-D-XYLOSIDE; 4-METHYLUMBELLIFERYL .BETA.-D-XYLOPYRANOSIDE; Q27894530; 4-METHYLUMBELLIFERYL .BETA.-D-XYLOPYRANOSIDE, (-)-; COUMARIN, 4-METHYL-7-(.BETA.-D-XYLOPYRANOSYLOXY)-; 7-(beta-D-Xylopyranosyloxy)-4-methyl-2H-1-benzopyran-2-one; 4-Methylumbelliferyl-beta-D-xylopyranoside, beta-xylosidase substrate; 4-METHYL-7-(.BETA.-D-XYLOPYRANOSYLOXY)-2H-1-BENZOPYRAN-2-ONE; 4-methyl-7-((2S,3R,4S,5R)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yloxy)-2H-chromen-2-one; 4-methyl-7-{[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxy}-2H-chromen-2-one; 4-methyl-7-(((2S,3R,4S,5R)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)-2H-chromen-2-one
Click to Show/Hide
|
||||
| Structure |
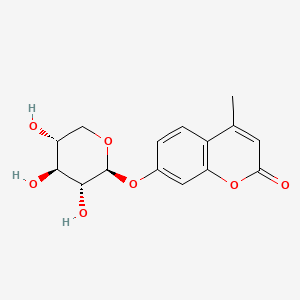 |
||||
| Formula |
C15H16O7
|
||||
| IUPAC Name |
4-methyl-7-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxychromen-2-one
|
||||
| Canonical SMILES |
CC1=CC(=O)OC2=C1C=CC(=C2)OC3C(C(C(CO3)O)O)O
|
||||
| InChI |
InChI=1S/C15H16O7/c1-7-4-12(17)22-11-5-8(2-3-9(7)11)21-15-14(19)13(18)10(16)6-20-15/h2-5,10,13-16,18-19H,6H2,1H3/t10-,13+,14-,15+/m1/s1
|
||||
| InChIKey |
JWIYLOHVJDJZOQ-KAOXEZKKSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Responsed Disease | Health | ICD-11: N.A. | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response regulation | The widely used sulfation inhibitor sodium chlorate and b-d-xyloside, which prevents proteoglycan glycosaminoglycan chain attachment, both reduced HS and CS, and exacerbated glutamate- and erastin-induced cell death, suggesting that extracellular matrix influenced oxytosis and ferroptosis. | |||
