Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0334)
| Name |
BMS536924
|
||||
|---|---|---|---|---|---|
| Synonyms |
BMS-536924; 468740-43-4; BMS536924; BMS 536924; 4-[[(2S)-2-(3-Chlorophenyl)-2-hydroxyethyl]amino]-3-[7-methyl-5-(4-morpholinyl)-1H-benzimidazol-2-yl]-2(1H)-pyridinone; 40E3AZG1MX; CHEMBL401930; (S)-4-((2-(3-chlorophenyl)-2-hydroxyethyl)amino)-3-(4-methyl-6-morpholino-1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one; CS-0117; HY-10262; KIN001-126; (3M)-4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-3-[4-methyl-6-(morpholin-4-yl)-1H-benzimidazol-2-yl]pyridin-2(1H)-one; 4-[[(2S)-2-(3-Chlorophenyl)-2-hydroxyethyl]amino]-3-(4-methyl-6-morpholin-4-yl-1H-benzimidazol-2-yl)-1H-pyridin-2-one; UNII-40E3AZG1MX; (S)-4-((2-(3-CHLOROPHENYL)-2-HYDROXYETHYL)AMINO)-3-(4-METHYL-6-MORPHOLINO-1H-BENZO(D)IMIDAZOL-2-YL)PYRIDIN-2(1H)-ONE; 4-(((2S)-2-(3-CHLOROPHENYL)-2-HYDROXYETHYL)AMINO)-3-(7-METHYL-5-(4-MORPHOLINYL)-1H-BENZIMIDAZOL-2-YL)-2(1H)-PYRIDINONE; N6I; MLS006011171; SCHEMBL4132577; SCHEMBL15974144; BDBM27879; CHEBI:91454; BCP02116; EX-A4566; NSC761760; s1012; BMS 536924 [WHO-DD]; AKOS024458339; AKOS025149514; CCG-269544; NSC-761760; NCGC00346460-02; NCGC00346460-04; NCGC00346460-05; NCGC00346460-06; AC-32997; AS-78828; SMR004702940; SW218124-2; D94990; BMS-536924, >=98% (HPLC); J-514217; BRD-K34581968-001-01-2; Q27163296; Q27258319; 2(1H)-PYRIDINONE, 4-(((2S)-2-(3-CHLOROPHENYL)-2-HYDROXYETHYL)AMINO)-3-(7-METHYL-5-(4-MORPHOLINYL)-1H-BENZIMIDAZOL-2-YL)-; 4-[[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]-3-[4-methyl-6-(4-morpholinyl)-1,3-dihydrobenzimidazol-2-ylidene]-2-pyridinone; 4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-3-[4-methyl-6-(morpholin-4-yl)-1H-1,3-benzodiazol-2-yl]-1,2-dihydropyridin-2-one; 4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-3-[7-methyl-5-(morpholin-4-yl)-1H-1,3-benzodiazol-2-yl]-1,2-dihydropyridin-2-one
Click to Show/Hide
|
||||
| Structure |
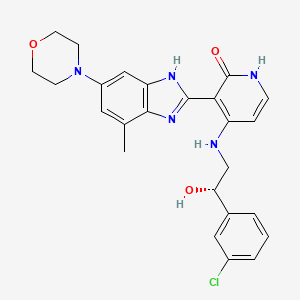 |
||||
| Formula |
C25H26ClN5O3
|
||||
| IUPAC Name |
4-[[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]-3-(4-methyl-6-morpholin-4-yl-1H-benzimidazol-2-yl)-1H-pyridin-2-one
|
||||
| Canonical SMILES |
CC1=CC(=CC2=C1N=C(N2)C3=C(C=CNC3=O)NCC(C4=CC(=CC=C4)Cl)O)N5CCOCC5
|
||||
| InChI |
InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1
|
||||
| InChIKey |
ZWVZORIKUNOTCS-OAQYLSRUSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | NCI-H522 cells | Non-small cell lung carcinoma | Homo sapiens | CVCL_1567 |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Response regulation | BMS536924, a dual inhibitor of insulin-like growth and insulin receptors, is a potent inhibitor of ferroptosis in non-small cell lung carcinoma. | |||
