Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0319)
| Name |
Doranidazole
|
||||
|---|---|---|---|---|---|
| Synonyms |
Doranidazole; 161903-10-2; Doranidazole [INN]; PR-69; 149838-23-3; PR-350; 4BLU68P76A; DTXSID3048817; 911XR034RX; NCGC00183013-01; RP 343; RP-343; 1,2,4-Butanetriol, 3-((2-nitro-1H-imidazol-1-yl)methoxy)-, (2R,3S)-; 1,2,4-Butanetriol, 3-((2-nitro-1H-imidazol-1-yl)methoxy)-, (S-(R*,S*))-; (2R,3S)-3-[(2-nitroimidazol-1-yl)methoxy]butane-1,2,4-triol; (2R,3S)-3-((2-nitro-1H-imidazol-1-yl)methoxy)butane-1,2,4-triol; (2RS,3SR)-3-{(2-Nitroimidazol-1-yl)methoxy}butane-1,2,4-triol; 1,2,4-Butanetriol, 3-[(2-nitro-1H-imidazol-1-yl)methoxy]-, (2R,3S)-; PR 350; UNII-4BLU68P76A; UNII-911XR034RX; (2RS,3SR)-3-((2-NITROIMIDAZOL-1-YL)METHOXY)BUTANE-1,2,4-TRIOL; 1-((2',3'-Dihydroxy-1'-hydroxymethyl)propoxy)methyl-2-nitroimidazole; 3-((2-Nitro-1H-imidazol-1-yl)methoxy)-1,2,4-butanetriol (R*,S*)-; DORANIDAZOLE [JAN]; SCHEMBL8279381; CHEMBL2107741; DTXCID0028743; Tox21_113294; 1,2,4-Butanetriol, 3-((2-nitro-1H-imidazol-1-yl)methoxy)-, (R*,S*)-; AKOS040751633; CAS-149838-23-3; 1-(1',3',4'-Trihydroxy-2'-butoxy)methyl-2-nitroimidazole; (2RSs,3SR)-3-((2-nitroimidazol-1-yl)methoxy)butane-1,2,4-triol; (2R,3S)-3-((2-NITRO-1H-IMIDAZOL-1-YL)METHOXY)-1,2,4-BUTANETRIOL; 1,2,4-BUTANETRIOL, 3-((2-NITRO-1H-IMIDAZOL-1-YL)METHOXY)-, (2R,3S)-REL-
Click to Show/Hide
|
||||
| Structure |
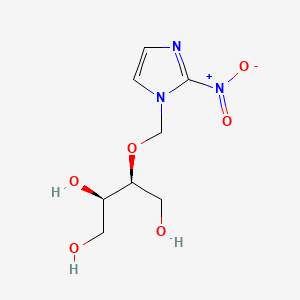 |
||||
| Formula |
C8H13N3O6
|
||||
| IUPAC Name |
(2R,3S)-3-[(2-nitroimidazol-1-yl)methoxy]butane-1,2,4-triol
|
||||
| Canonical SMILES |
C1=CN(C(=N1)[N+](=O)[O-])COC(CO)C(CO)O
|
||||
| InChI |
InChI=1S/C8H13N3O6/c12-3-6(14)7(4-13)17-5-10-2-1-9-8(10)11(15)16/h1-2,6-7,12-14H,3-5H2/t6-,7+/m1/s1
|
||||
| InChIKey |
FIITXXIVUIXYMI-RQJHMYQMSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mGICs (Mouse glioma initiating cells) | ||||
| mGSCs (Mouse glioma stem cells) | |||||
| In Vivo Model |
Female C57BL/6J mice (age 6-8 weeks) were anesthetized and placed into a stereotactic apparatus (David Kopf Instruments, Tujunga, CA). One thousand viable GSC-H cells were injected into the right hemisphere at a position 2 mm lateral to the bregma and 3 mm below the brain surface. After 10 days, animals were exposed to 15 Gy (radiation dose rate, 1.45 Gy/min) with or without prior injection of doranidazole (200 mg/kg, i.p.). Radiation was confined to the brain by protection of the body with a lead shield.
Click to Show/Hide
|
||||
| Response regulation | Doranidazole and misonidazole, but not metronidazole, manifested radiation-independent cytotoxicity for hypoxic glioma stem cells (GSCs) that was mediated by ferroptosis induced partially through blockade of mitochondrial complexes I and II and resultant metabolic alterations in oxidative stress responses. | ||||
