Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0315)
| Name |
Poricoic acid A
|
||||
|---|---|---|---|---|---|
| Synonyms |
Poricoic acid A; 137551-38-3; Poricoic acid A(F); CHEBI:68353; (2R)-2-[(2R,3R,3aR,6S,7S,9bR)-6-(2-carboxyethyl)-2-hydroxy-3a,6,9b-trimethyl-7-prop-1-en-2-yl-1,2,3,4,7,8-hexahydrocyclopenta[a]naphthalen-3-yl]-6-methyl-5-methylideneheptanoic acid; PoricoicacidA; CHEMBL461483; SCHEMBL22200216; DTXSID401346653; HY-N2995; NSC715080; AKOS027326667; NSC-715080; AC-34216; MS-29260; NCI60_039753; CS-0023654; A903857; Q27136850; 16.alpha.-Hydroxy-3,7,9(11),24(24(sup 1))-tetraene-3,21-dioic acid; (2R)-2-[(2R,3R,3aR,6S,7S,9bR)-6-(2-carboxyethyl)-2-hydroxy-3a,6,9b-trimethyl-7-(prop-1-en-2-yl)-2,3,3a,4,6,7,8,9b-octahydro-1H-cyclopenta[a]naphthalen-3-yl]-6-methyl-5-methylideneheptanoic acid; (2R)-2-[(2R,3R,3aR,6S,7S,9bR)-6-(2-carboxyethyl)-2-hydroxy-7-isopropenyl-3a,6,9b-trimethyl-1,2,3,4,7,8-hexahydrocyclopenta[a]naphthalen-3-yl]-6-methyl-5-methylene-heptanoic acid; (2R,3R,3aR,6S,7S,9bR)-3-[(1R)-1-Carboxy-5-methyl-4-methylenehexyl]-2,3,3a,4,6,7,8,9b-octahydro-2-hydroxy-3a,6,9b-trimethyl-7-(1-methylethenyl)-1H-benz[e]indene-6-propanoic acid; (R)-2-[(2R,3R,3aR,6S,7S,9bR)-6-(2-Carboxy-ethyl)-2-hydroxy-7-isopropenyl-3a,6,9b-trimethyl-2,3,3a,4,6,7,8,9b-octahydro-1H-benz[e]inden-3-yl]-6-methyl-5-methylene-heptanoic acid
Click to Show/Hide
|
||||
| Structure |
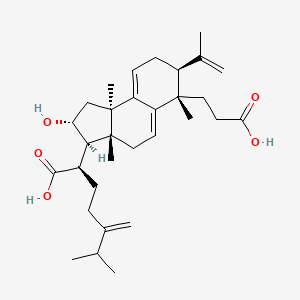 |
||||
| Formula |
C31H46O5
|
||||
| IUPAC Name |
(2R)-2-[(2R,3R,3aR,6S,7S,9bR)-6-(2-carboxyethyl)-2-hydroxy-3a,6,9b-trimethyl-7-prop-1-en-2-yl-1,2,3,4,7,8-hexahydrocyclopenta[a]naphthalen-3-yl]-6-methyl-5-methylideneheptanoic acid
|
||||
| Canonical SMILES |
CC(C)C(=C)CCC(C1C(CC2(C1(CC=C3C2=CCC(C3(C)CCC(=O)O)C(=C)C)C)C)O)C(=O)O
|
||||
| InChI |
InChI=1S/C31H46O5/c1-18(2)20(5)9-10-21(28(35)36)27-25(32)17-31(8)24-12-11-22(19(3)4)29(6,15-14-26(33)34)23(24)13-16-30(27,31)7/h12-13,18,21-22,25,27,32H,3,5,9-11,14-17H2,1-2,4,6-8H3,(H,33,34)(H,35,36)/t21-,22+,25-,27+,29+,30-,31+/m1/s1
|
||||
| InChIKey |
KVAQLXUMUVEKGR-SMFZDKLCSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Mature T-cell lymphoma | ICD-11: 2A90 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | Jurkat cells | T acute lymphoblastic leukemia | Homo sapiens | CVCL_0065 | |
| MOLT-3 cells | T-lymphoblastic leukemia | Homo sapiens | CVCL_0624 | ||
| RPMI-8402 cells | Acute lymphoblastic leukemia | Homo sapiens | CVCL_1667 | ||
| RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | ||
| HK-2 cells | Normal | Homo sapiens | CVCL_0302 | ||
| CAM191 cells | Normal | Homo sapiens | CVCL_BS93 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
BALB/c Nude Mice (4-6 weeks old) were purchased from Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China) and then subcutaneously injection with JURKAT cells (1 x 107) suspended in 100 uL PBS. Tumor volume was measured once a week with a caliper according to the formula: 1/2 x length x width2. After tumors reached 100-200 mm3(at day 4), the mice were randomly assigned to three groups: the Control group, low dose of PAA (5 mg/kg) and high dose of PAA (10 mg/kg). PAA was administered through i.p. injection once a day at 5 or 10 mg/kg body weight. Mice in normal control group were given equal amounts of vehicle (saline). Body weights of mice were measured once a week during PAA treatments. After 4 weeks, blood samples were collected from each group of mice, and tumor tissues were removed and weighed for further analysis.
Click to Show/Hide
|
||||
| Response regulation | Poricoic acid A could significantly inhibit T-cell acute lymphoblastic leukemia (T-ALL) progression by inducing G2/M phase, apoptosis, mitochondrial dysfunction, ROS generation, autophagy and ferroptosis without detectable side effects both in vitro and in vivo. | ||||
