Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0305)
| Name |
U0126
|
||||
|---|---|---|---|---|---|
| Synonyms |
U0126; 109511-58-2; U-0126; 1,4-Diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene; U 0126; 218601-62-8; (2Z,3Z)-2,3-bis(amino((2-aminophenyl)thio)methylene)succinonitrile; (2Z,3Z)-bis{amino[(2-aminophenyl)sulfanyl]methylidene}butanedinitrile; FT-1069-1; CHEBI:64208; 8027P94HLL; 109511-58-2 (free); 2,3-Bis(amino((2-aminophenyl)thio)methylene)succinonitrile; (2z)-Bis{amino[(2-Aminophenyl)sulfanyl]methylidene}butanedinitrile; (2Z,3Z)-2,3-Bis[amino[(2-aminophenyl)thio]methylene]butanedinitrile; (2Z,3Z)-bis{amino[(2-aminophenyl)sulfanyl]methylene}succinonitrile; Butanedinitrile, bis(amino((2-aminophenyl)thio)methylene)-, (2Z,3Z)-; 1,4-Diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene; (2Z,3Z)-2,3-bis[amino-(2-aminophenyl)sulfanylmethylidene]butanedinitrile; 1,4-Diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene; BiomolKI_000002; BiomolKI2_000012; BMK1-B2; BSPBio_001224; CHEMBL34704; UNII-8027P94HLL; GTPL5282; Succinonitrile, bis(amino(o-aminophenylthio)methylene)-; CHEBI:90693; CHEBI:91463; Butanedinitrile, bis(amino((2-aminophenyl)thio)methylene)-; DTXSID10892034; HMS1362N05; HMS1792N05; HMS1990N05; HMS3403N05; HMS3414K05; HMS3678K05; AMY31125; BCP01851; EX-A1754; HB2246; HY-12031A; (2Z,3Z)-2,3-bis[amino-(2-aminophenyl)sulfanyl-methylene]butanedinitrile; (2Z,3Z)-bis({amino[(2-aminophenyl)sulfanyl]methylidene})butanedinitrile; AKOS024456414; AKOS040744815; CCG-100606; IDI1_002207; SMP2_000197; NCGC00025029-02; NCGC00025029-03; NCGC00025029-04; CS-0003351; U 126; A846574; A906530; SR-01000597365; J-002297; Q7863562; SR-01000597365-1; BRD-K18787491-001-04-5; BRD-K46419649-001-01-8; U-0126 is known as an MEK inhibitor and AMPK activator.; Butanedinitrile,2,3-bis[amino[(2-aminophenyl)thio]methylene]-; 5BM
Click to Show/Hide
|
||||
| Structure |
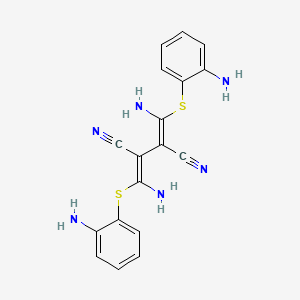 |
||||
| Formula |
C18H16N6S2
|
||||
| IUPAC Name |
(2Z,3Z)-2,3-bis[amino-(2-aminophenyl)sulfanylmethylidene]butanedinitrile
|
||||
| Canonical SMILES |
C1=CC=C(C(=C1)N)SC(=C(C#N)C(=C(N)SC2=CC=CC=C2N)C#N)N
|
||||
| InChI |
InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8H,21-24H2/b17-11+,18-12+
|
||||
| InChIKey |
DVEXZJFMOKTQEZ-JYFOCSDGSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Intracerebral hemorrhage | ICD-11: 8B00 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks old) were purchased from Charles River Laboratories. For intraperitoneal injections, mice received daily intraperitoneal injections of 25 mg/kg U0126 or vehicle for 7 d (n = 13 for sham+vehicle, n = 17 for ICH+vehicle, n = 16 for ICH + 25 mg/kg U0126). Four ICH and six ICH+U0126 animals died from the surgery. For intracerebroventricular injections, mice received a single intracerebroventricular injection of 12 ug U0126 or vehicle (n = 16 per group) 2 h after ICH.
Click to Show/Hide
|
||||
| Response regulation | Ferroptosis is an iron-dependent mechanism of regulated necrosis that has been linked to hemorrhagic stroke. The mitogen-activated protein (MAP) kinase kinase (MEK) inhibitor U0126 inhibits persistent ERK1/2 phosphorylation and ferroptosis. | ||||
