Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0231)
| Name |
Kaempferide
|
||||
|---|---|---|---|---|---|
| Synonyms |
Kaempferide; 491-54-3; Kaempferid; 4'-Methylkaempferol; 4'-O-Methylkaempferol; Kaempferol 4'-methyl ether; 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one; 3,5,7-trihydroxy-2-(4-methoxyphenyl)chromen-4-one; Campheride; Kaempferol 4'-O-methyl ether; Kaemperide; Kampheride; 5,7-Dihydroxy-4'-methoxyflavonol; 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-methoxyphenyl)-; 4'-Methoxy-3,5,7-trihydroxyflavone; NSC 407294; KAMPFERIDE; NSC-407294; 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4-benzopyrone; CHEMBL40919; 508XL61MPD; CHEBI:6099; FLAVANONE, 4'-METHOXY-3,5,7-TRIHYDROXY-; NSC407294; Kempferide; 4'-Methoxykaempferol; EINECS 207-738-4; BRN 0305378; UNII-508XL61MPD; Kaemferide; Flavone, 3,5,7-trihydroxy-4'-methoxy-; Kaempferide (AS); 3 5 7-trihydroxy-4'-methoxyflavone; 3,5,7-Trihydroxy-4'-methoxyflavone; 4'-Methoxy-3',5,7-trihydroxyflavone; Kaempferol 4''-methyl ether; 5-18-05-00253 (Beilstein Handbook Reference); SCHEMBL426774; Kaempferide, analytical standard; DTXSID9034155; AMY5862; SQFSKOYWJBQGKQ-UHFFFAOYSA-N; HMS3746E09; BCP20573; BDBM50084978; LMPK12110563; MFCD00016771; s3879; Flavone,5,7-trihydroxy-4'-methoxy-; AKOS015903441; 3,5,7-Trihydroxy-4'-methoxy-Flavone; CCG-267464; CS-0957; 4'-Methoxy-3,5,7-trihydroxy-Flavanone; NCGC00379085-03; AC-30805; BS-16895; HY-15449; FT-0625213; K0057; WLN: T66 BO EVJ CR DO1& DQ GQ IQ; A827662; Q3116775; 1.3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4-benzopyrone; 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one #; 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one; 4H-1-Benzopyran-4-one,5,7-trihydroxy-2-(4-methoxyphenyl)-; 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one, 9CI
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
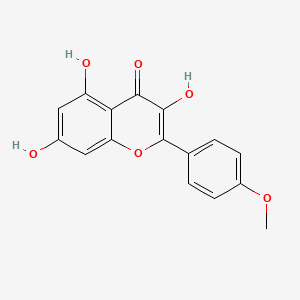 |
||||
| Formula |
C16H12O6
|
||||
| IUPAC Name |
3,5,7-trihydroxy-2-(4-methoxyphenyl)chromen-4-one
|
||||
| Canonical SMILES |
COC1=CC=C(C=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O
|
||||
| InChI |
InChI=1S/C16H12O6/c1-21-10-4-2-8(3-5-10)16-15(20)14(19)13-11(18)6-9(17)7-12(13)22-16/h2-7,17-18,20H,1H3
|
||||
| InChIKey |
SQFSKOYWJBQGKQ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Responsed Disease | Nervous system disease | ICD-11: 8E7Z | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response regulation | The ethanol extracts of Brazilian green propolis help to prevent oxidative stress-related neuronal cell death that is involved in the pathogenesis of several neurodegenerative diseases. Among the primary constituents of ethanol extracts of Brazilian green propolis, only artepillin C, kaempferide, and kaempferol demonstrated neuroprotective effects against oxytosis/ferroptosis. | |||
