Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0209)
| Name |
Neratinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Neratinib; 698387-09-6; HKI-272; Neratinib (HKI-272); Nerlynx; HKI 272; PB-272; JJH94R3PWB; (2E)-N-[4-[[3-chloro-4-[(pyridin-2-yl)methoxy]phenyl]amino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide; (E)-N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide; CHEMBL180022; CDP-820; 698387-09-6 (free base); HKI272; WAY-179272; (2E)-N-(4-{[3-chloro-4-(pyridin-2-ylmethoxy)phenyl]amino}-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide; (E)-N-(4-((3-Chloro-4-(pyridin-2-ylmethoxy)phenyl)amino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide; 876310-02-0; (E)-N-[4-[3-chloro-4-(pyridin-2-ylmethoxy)anilino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide; N-(4-(3-Chloro-4-(2-pyridinylmethoxy)anilino)-3-cyano-7-ethoxy-6-quinolyl)-4-(dimethylamino)-2-butenamide; (2e)-n-(4-((3-chloro-4-((pyridin-2-yl)methoxy)phenyl)amino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide; 2-Butenamide, N-(4-((3-chloro-4-(2-pyridinylmethoxy)phenyl)amino)-3-cyano-7-ethoxy-6-quinolinyl)-4-(dimethylamino)-, (2E)-; Neratinib [USAN]; Neratinib(HKI-272); Neratinib [USAN:INN]; UNII-JJH94R3PWB; Neratinib- Bio-X; (2E)-N-[4-({3-chloro-4-[(pyridin-2-yl)methoxy]phenyl}amino)-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide; PB 272; NERATINIB [INN]; NERATINIB [MI]; Neratinib (USAN/INN); Neratinib - HKI-272; NERATINIB [MART.]; NERATINIB [WHO-DD]; SCHEMBL571762; SCHEMBL571763; GTPL5686; CHEBI:61397; AMY9255; DTXSID70220132; EX-A062; BCPP000151; BDBM50161957; MFCD09752958; NSC757439; NSC800803; s2150; WAY-179272-B; AKOS005146340; AKOS025149637; BCP9000984; CCG-270036; DB11828; NSC-757439; NSC-800803; NCGC00241101-01; NCGC00241101-03; NCGC00241101-09; AC-25073; AS-16279; BN164645; HY-32721; N1062; EC-000.2260; A25338; D08950; EN300-7386009; Q-101402; Q6995920; BRD-K85606544-001-01-8; (2E)-N-[4-[[3-Chloro-4-(2-pyridinylmethoxy)phenyl]amino]-3-cyano-7-ethoxy-6-quinolinyl]-4-(dimethylamino)-2-butenamide; (E)-4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(pyridin-2-ylmethoxy)-phenylamino]-3-cyano-7-ethoxy-quinolin-6-yl}-amide; 4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(pyridin-2-ylmethoxy)-phenylamino]-3-cyano-7-ethoxy-quinolin-6-yl}-amide; HKI-272; PB272;;(2E)-N-[4-[[3-chloro-4-[(pyridin-2-yl)methoxy]phenyl]amino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide;HKI-272; N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide; N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)butanamide
Click to Show/Hide
|
||||
| Structure |
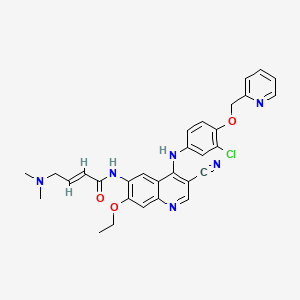 |
||||
| Formula |
C30H29ClN6O3
|
||||
| IUPAC Name |
(E)-N-[4-[3-chloro-4-(pyridin-2-ylmethoxy)anilino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide
|
||||
| Canonical SMILES |
CCOC1=C(C=C2C(=C1)N=CC(=C2NC3=CC(=C(C=C3)OCC4=CC=CC=N4)Cl)C#N)NC(=O)C=CCN(C)C
|
||||
| InChI |
InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+
|
||||
| InChIKey |
JWNPDZNEKVCWMY-VQHVLOKHSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
| In Vitro Model | 67NR cells | Breast carcinoma | Mus musculus | CVCL_9723 | |
| 4T1.2 cells | Breast carcinoma | Mus musculus | CVCL_GR32 | ||
| 4T1 cells | Mammary carcinoma | Mus musculus | CVCL_0125 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| BT-474 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0179 | ||
| SK-BR-3 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| In Vivo Model |
Female BALB/C mice (5/box) were maintained in a specific pathogen-free environment. For initial characterisation of metastatic spread, TBCP-1 cells (5 x 105) were injected into the left cardiac ventricle of 6-8-week-old female BALB/C mice. The mice were monitored daily and sacrificed after 3 weeks or earlier if signs of metastases became apparent (weight loss > 10%, ruffled fur, lethargy, rapid breathing).
Click to Show/Hide
|
||||
| Response regulation | Neratinib promotes a non-apoptotic form of cell death termed ferroptosis. Importantly, metastasis assays demonstrate that neratinib potently inhibits breast cancer growth and metastasis, including to the brain, and prolongs survival, particularly when used as a neoadjuvant therapy. | ||||
