Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0197)
| Name |
Romidepsin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Romidepsin; Depsipeptide; FK228; Chromadax; 128517-07-7; Istodax; Antibiotic FR 901228; FR901228; FK 228; FK-228; FR 901228; FR-901228; NSC-630176; C24H36N4O6S2; NSC 630176; CHEMBL343448; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; CHEBI:61080; NSC630176; NSC754143; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; Istodax (TN); Romidepsin [USAN]; CX3T89XQBK; HDInhib_000006; romidepsina; romidepsine; romidepsinum; OXA-12,8,20,23-TETRAZABICYCLO[8.7.6]TRICOSANE, CYCLIC PEPTIDE DERIV.; Romidepsin (FK228); Romidepsin; FK-228; ROMIDEPSIN [MI]; FK-901228; ROMIDEPSIN [INN]; ROMIDEPSIN [JAN]; ROMIDEPSIN [VANDF]; Probes1_000153; Probes2_000337; ROMIDEPSIN [MART.]; ROMIDEPSIN [WHO-DD]; DEPSIPEPTIDE [WHO-DD]; Romidepsin (JAN/USAN/INN); SCHEMBL677497; GTPL7006; ROMIDEPSIN [ORANGE BOOK]; Romidepsin, >=98% (HPLC); BDBM19151; Romidepsin (FK228 ,depsipeptide); (1S,4S,7Z,10S,16E,21R)-7-Ethylidene-4,21-bis(1-methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; Cyclo((2Z)-2-amino-2-butenoyl-L-valyl-(3S,4E)-3-hydroxy-7-mercapto-4-heptenoyl-D-valyl-D-cysteinyl), cyclic (3->5)-disulfide; HB1386; MFCD18433404; s3020; API0005301; CS-0985; DB06176; NSC-754143; HY-15149; D06637; AB01273968-01; EN300-22844947; SR-01000941579; Q7363205; SR-01000941579-1; L-Valine,3-didehydro-2- aminobutanoyl-,.xi.-lactone, cyclic (1.fwdarw.2)-disulfide; L-Valine,3-didehydro-2-aminobutanoyl-,.xi.-lactone, cyclic (1.fwdarw.2)-disulfide; (1S,4S,7Z,10S,16E,21R)-7- ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23- tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(1methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16ene-3,6,9,19,22-pentone; (E)-(1S,10S,21R)-7-[(Z)-Ethylidene]-4,21-diisopropyl-2- oxa-12,13-dithia-5,8,20,23- tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; (E)-(1S,10S,21R)-7-[(Z)-Ethylidene]-4,21-diisopropyl-2- oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; CYCLO((2Z)-2-AMINO-2-BUTENOYL-L-VALYL-(3S,4E)-3-HYDROXY-7-MERCAPTO-4-HEPTENOYL-D-VALYL-D-CYSTEINYL), CYCLIC (3->5)-DISULPHIDE; Cyclo[(2Z)-2-amino-2-butenoyl-L-val yl-(3S,4E)-3-hydroxy-7-mercapto-4-heptenoyl-D-valy l-D-cysteinyl], cyclic (3-5) disulfide; Cyclo[(2Z)-2-amino-2-butenoyl-L-valyl-(3S,4E)-3-hydroxy-7-mercapto-4-heptenoyl-D-valyl-D-cysteinyl], cyclic (3-5) disulfide
Click to Show/Hide
|
||||
| Structure |
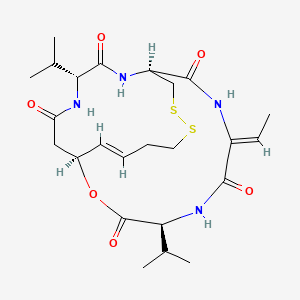 |
||||
| Formula |
C24H36N4O6S2
|
||||
| IUPAC Name |
(1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
|
||||
| Canonical SMILES |
CC=C1C(=O)NC(C(=O)OC2CC(=O)NC(C(=O)NC(CSSCCC=C2)C(=O)N1)C(C)C)C(C)C
|
||||
| InChI |
InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1
|
||||
| InChIKey |
OHRURASPPZQGQM-GCCNXGTGSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 2 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Responsed Disease | Adrenocortical carcinoma | ICD-11: 2D11 | ||
| Responsed Regulator | Histone deacetylase 1 (HDAC1) | Suppressor | ||
| Pathway Response | Cell adhesion molecules | hsa04514 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | SW13 cells | Adrenal cortex carcinoma | Homo sapiens | CVCL_0542 |
| Response regulation | HDAC inhibitor Romidepsin converted SW13+ cells also had reduced mRNA expression of the mitochondrial ROS detoxifier superoxide dismutase 2 (SOD2), and the tumor suppressor p53. HDAC inhibitor treatment synergistically increased adrenocortical carcinoma cell death following induction of ferroptosis. | |||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Responsed Disease | Adrenocortical carcinoma | ICD-11: 2D11 | ||
| Responsed Regulator | Cellular tumor antigen p53 (TP53) | Driver | ||
| Pathway Response | Cell adhesion molecules | hsa04514 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | SW13 cells | Adrenal cortex carcinoma | Homo sapiens | CVCL_0542 |
| Response regulation | HDAC inhibitor Romidepsin converted SW13+ cells also had reduced mRNA expression of the mitochondrial ROS detoxifier superoxide dismutase 2 (SOD2), and the tumor suppressor p53. HDAC inhibitor treatment synergistically increased adrenocortical carcinoma cell death following induction of ferroptosis. | |||
