Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0189)
| Name |
Dyclonine
|
||||
|---|---|---|---|---|---|
| Synonyms |
dyclonine; Dyclocaine; 586-60-7; Dyclonin; Dyclocainum; Diclonina; Dycloninum; 1-(4-butoxyphenyl)-3-(piperidin-1-yl)propan-1-one; Dycloninum [INN-Latin]; Diclonina [INN-Spanish]; 4'-Butoxy-3-piperidinopropiophenone; 1-(4-butoxyphenyl)-3-piperidin-1-ylpropan-1-one; 3-Piperidino-4'-butoxypropiophenone; 1-Propanone, 1-(4-butoxyphenyl)-3-(1-piperidinyl)-; Dyclonine (INN); Diclonia; 2-(1-piperidyl)ethyl p-butoxyphenyl ketone; 4-butoxy-beta-piperidinopropiophenone; 1-(4-Butoxyphenyl)-3-(1-piperidinyl)-1-propanone; 4-n-butoxy-beta-(1-piperidyl)propiophenone; CHEBI:4724; PROPIOPHENONE, 4'-BUTOXY-3-PIPERIDINO-; 078A24Q30O; DYCLONINE [INN]; Dyclonine [INN:BAN]; NCGC00016498-01; CAS-536-43-6; BRN 0224037; UNII-078A24Q30O; Dyclone (Salt/Mix); Tanaclone (Salt/Mix); Spectrum_001016; DYCLONINE [MI]; Prestwick0_000264; Prestwick1_000264; Prestwick2_000264; Prestwick3_000264; Spectrum2_001013; Spectrum3_000410; Spectrum4_000529; Spectrum5_000951; DYCLONINE [VANDF]; DYCLONINE [WHO-DD]; SCHEMBL25773; BSPBio_000108; BSPBio_001940; KBioGR_001137; KBioSS_001496; DivK1c_000632; 1-propanone, 1-(4-butoxyphenyl)-3-(1-piperidinyl); SPBio_001165; SPBio_002327; 1-(4-Butoxy-phenyl)-3-piperidin-1-yl-propan-1-one; BPBio1_000120; GTPL7173; CHEMBL1201217; DTXSID6047864; KBio1_000632; KBio2_001496; KBio2_004064; KBio2_006632; KBio3_001160; BZEWSEKUUPWQDQ-UHFFFAOYSA-N; NINDS_000632; EX-A5489; AM9638; STK524544; AKOS000505031; DB00645; IDI1_000632; NCGC00016498-02; NCGC00016498-03; NCGC00016498-04; NCGC00016498-05; AC-12286; SBI-0051358.P003; AB00053467; FT-0660914; C07881; D07881; AB00053467_13; AB00053467_14; EN300-25300221; Q425386; BRD-K72259270-003-05-8; BRD-K72259270-003-15-7; N8R
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
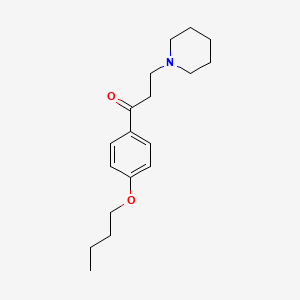 |
||||
| Formula |
C18H27NO2
|
||||
| IUPAC Name |
1-(4-butoxyphenyl)-3-piperidin-1-ylpropan-1-one
|
||||
| Canonical SMILES |
CCCCOC1=CC=C(C=C1)C(=O)CCN2CCCCC2
|
||||
| InChI |
InChI=1S/C18H27NO2/c1-2-3-15-21-17-9-7-16(8-10-17)18(20)11-14-19-12-5-4-6-13-19/h7-10H,2-6,11-15H2,1H3
|
||||
| InChIKey |
BZEWSEKUUPWQDQ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Head neck squamous cell carcinoma | ICD-11: 2D60 | |||
| Responsed Regulator | Aldehyde dehydrogenase, dimeric NADP-preferring (ALDH3A1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | OSC-19 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_3086 | |
| HSC-2 cells | Oral cavity squamous cell carcinoma | Homo sapiens | CVCL_1287 | ||
| HSC-3 cells | Oral squamous cell carcinoma | Homo sapiens | CVCL_1288 | ||
| HSC-4 cells | Cervical lymph node | Homo sapiens | CVCL_1289 | ||
| SCC-25 cells | Squamous carcinoma | Homo sapiens | CVCL_1682 | ||
| DMS114 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1174 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| 4T1 cells | Mammary carcinoma | Mus musculus | CVCL_0125 | ||
| In Vivo Model |
HSC-2 or K19-Wnt1/C2mE-KP cells (2 x 106 cells per site) were implanted subcutaneously in the flank of athymic nude mice (CLEA Japan) or C57BL6 mice (CLEA Japan), respectively. The mice were then injected intraperitoneally with physiological saline or sulfasalazine (350 mg/kg per day), or with combinations of physiological saline, sulfasalazine (400 mg/kg per day), and dyclonine hydrochloride (5 mg/kg per day).
Click to Show/Hide
|
||||
| Response regulation | Sulfasalazine-resistant head and neck squamous cell carcinoma (HNSCC) cells were found to highly express ALDH3A1 and knockdown of ALDH3A1 rendered these cells sensitive to sulfasalazine. The combination of dyclonine and sulfasalazine cooperatively suppressed the growth of highly ALDH3A1-expressing HNSCC or gastric tumors that were resistant to sulfasalazine monotherapy. | ||||
