Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0149)
| Name |
Gingerol
|
||||
|---|---|---|---|---|---|
| Synonyms |
6-Gingerol; gingerol; 23513-14-6; [6]-Gingerol; (6)-Gingerol; (S)-(+)-[6]Gingerol; (S)-(6)-Gingerol; (S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)decan-3-one; (5S)-5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)decan-3-one; 1391-73-7; (+)-[6]-Gingerol; (5S)-5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-decanone; 3-Decanone, 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-, (5S)-; UNII-925QK2Z900; CHEBI:10136; (+)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-decanone; 925QK2Z900; C17-H26-O4; (S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-decanone; CHEMBL402978; (5S)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl)decan-3-one; 3-Decanone, 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-, (S)-; 3-Decanone, 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-, (S)-(+)-; 5-Hydroxy-1-(4'-hydroxy-3'-methoxyphenyl)-3-decanone; BDBM50317427; (S)-(+)-[6]Gingerol;6-Gingerol; MFCD00210507; CBiol_001786; SCHEMBL32102; BSPBio_001347; KBioGR_000067; KBioSS_000067; (6)-GINGEROL [MI]; CHEMBL446043; GTPL2428; DTXSID3041035; KBio2_000067; KBio2_002635; KBio2_005203; KBio3_000133; KBio3_000134; Bio1_000072; Bio1_000561; Bio1_001050; Bio2_000067; Bio2_000547; HMS1361D09; HMS1791D09; HMS1989D09; HMS3402D09; SRCG-00265; [6]-Gingerol, analytical standard; BDBM50275147; CA-422; s3836; [6]-Gingerol, >=98% (HPLC); AKOS015888215; CCG-208033; CCG-208214; CS-6333; IDI1_033817; NCGC00163131-01; NCGC00163131-02; NCGC00163131-03; AC-33934; DS-12369; HY-14615; 6-GINGEROL (CONSTITUENT OF GINGER); C10462; 6-GINGEROL (CONSTITUENT OF GINGER) [DSC]; A816759; Q421081; SR-05000002341; Q-100300; SR-05000002341-2; W-205525; BRD-K26117720-001-02-2; (S)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl-3-decanone; [1-(4''-hydroxy-3''-methoxyphenyl)-5-hydroxy-3-decanone]
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
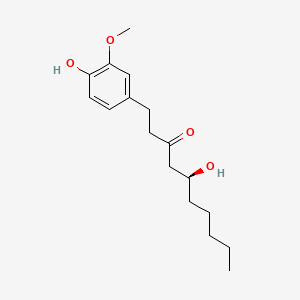 |
||||
| Formula |
C17H26O4
|
||||
| IUPAC Name |
(5S)-5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)decan-3-one
|
||||
| Canonical SMILES |
CCCCCC(CC(=O)CCC1=CC(=C(C=C1)O)OC)O
|
||||
| InChI |
InChI=1S/C17H26O4/c1-3-4-5-6-14(18)12-15(19)9-7-13-8-10-16(20)17(11-13)21-2/h8,10-11,14,18,20H,3-7,9,12H2,1-2H3/t14-/m0/s1
|
||||
| InChIKey |
NLDDIKRKFXEWBK-AWEZNQCLSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Responsed Regulator | Ubiquitin carboxyl-terminal hydrolase 14 (USP14) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
BALB/cNude (6-8 weeks of age) mice were purchased from Hangzhou Ziyuan Experimental Animal Technology Co. Ltd. (SYXK-20180049) for this study. The mice were housed under specific pathogen-free conditions at 23 and given free access to food and water. The left flank of mice was subcutaneously inoculated with A549 tumor-cell suspension (5 x 106 cells/100uL) to prepare A549 tumor xenografts. Three days after tumor cell inoculation, the mice were divided into three groups (n = 8): Con group (control group, no treatment), L-Gin group (0.25 mg/kg/day 6-Gingerol), H-Gin group (0.5 mg/kg/day 6-Gingerol), which were administered orally daily until the end of the experiments. Mice were killed when their minor axis of tumors were longer than 20 mm.
Click to Show/Hide
|
||||
| Response regulation | 6-Gingerol inhibits lung cancer cell growth via suppression of USP14 expression and its downstream regulation of autophagy-dependent ferroptosis, revealing the function and efficacy of 6-Gingerol as a therapeutic compound in A549 and its possible mechanism of action. | ||||
