Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0142)
| Name |
Dexrazoxane
|
||||
|---|---|---|---|---|---|
| Synonyms |
Dexrazoxane; 24584-09-6; Zinecard; (S)-4,4'-(Propane-1,2-diyl)bis(piperazine-2,6-dione); Cardioxane; ICRF-187; Dextrorazoxane; Dexrazoxanum; Dexrazoxano; Dexrazoxanum [INN-Latin]; Dexrazoxano [INN-Spanish]; ADR-529; Desrazoxane; Eucardion; (+)-(S)-4,4'-Propylenedi-2,6-piperazinedione; 4-[(2S)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione; ADR 529; ICRF 187; Razoxane, d-; Razoxane, (s)-; Dexrazone; (+)-1,2-Bis(3,5-dioxo-1-piperazinyl)propane; NSC-169780; CHEBI:50223; Razoxanum [INN-Latin]; Razoxana [INN-Spanish]; dyzoxane; DTXSID3040647; 2,6-Piperazinedione, 4,4'-[(1S)-1-methyl-1,2-ethanediyl]bis-; 2,6-Piperazinedione, 4,4'-(1-methyl-1,2-ethanediyl)bis-, (S)-; NSC 169780; (S)-(+)-1,2-Bis(3,5-dioxopiperazin-1-yl)propane; 048L81261F; 4-[(2S)-1-(3,5-dioxopiperazin-1-yl)propan-2-yl]piperazine-2,6-dione; (S)-1,2-Bis(3,5-dioxo-1-piperazinyl)propane; DTXCID1020647; (+)-dexrazoxane; CAS-24584-09-6; 2,6-Piperazinedione, 4,4'-(1-methyl-1,2-ethanediyl)bis-, (+)-; HSDB 7319; (+)-1,2-Bis(3,5-dioxopiperazin-1-yl)propane; 2,6-Piperazinedione, 4,4'-propylenedi-, (+)-; SR-01000883995; NSC169780; BRN 5759131; Soluble ICRF (L-isosomer); Dexrazoxane [USAN:INN:BAN]; CCRIS 9394; UNII-048L81261F; NCGC00164737-01; Dexrazoxane- Bio-X; MFCD00866449; DEXRAZOXANE [INN]; DEXRAZOXANE [JAN]; DEXRAZOXANE [HSDB]; DEXRAZOXANE [USAN]; DEXRAZOXANE [VANDF]; CHEMBL1738; DEXRAZOXANE [MART.]; SCHEMBL18400; DEXRAZOXANE [WHO-DD]; MLS006010158; BIDD:GT0068; Dexrazoxane (JAN/USAN/INN); DEXRAZOXANE [EMA EPAR]; 4,4'-(2S)-propane-1,2-diyldipiperazine-2,6-dione; GTPL7330; Dexrazoxane, >=95% (HPLC); (S)-4,4'-(Propane-1,2-diyl)-bis(piperazine-2,6-dione); RAZOXANE (+)-FORM [MI]; AMY39004; HY-B0581; Tox21_112256; 2, 4,4'-propylenedi-, (+)-; BDBM50586360; s5651; AKOS015896392; Tox21_112256_1; (+)-1,5-dioxopiperazin-1-yl)propane; CCG-267131; DB00380; DS-1394; NCGC00263544-01; NCGC00263544-02; BD164365; Dexrazoxane, analytical reference material; NCI60_001367; SMR002529680; (S)-(+)-1,5-dioxopiperazin-1-yl)propane; D4227; D03730; AB01273932-01; AB01273932-02; AB01273932_03; EN300-7356981; 2,4,4'-[(1S)-1-methyl-1,2-ethanediyl]bis-; A817380; Q524995; 2,4,4'-(1-methyl-1,2-ethanediyl)bis-, (S)-; J-015579; J-520219; SR-01000883995-1; SR-01000883995-2; SR-01000883995-5; (S)-4,4'-(propane-1,2-diyl)dipiperazine-2,6-dione; BRD-K07265709-003-01-5; 4,4'-[(2S)-1,2-Propanediyl]di(2,6-piperazinedione); 4,4'-[(1S)-1-Methyl-1,2-ethanediyl]bis-2,6-piperazinedione; 6-hydroxy-4-[(2S)-2-(5-hydroxy-3-oxo-1,2,3,6-tetrahydropyrazin-1-yl)propyl]-2,3,4,5-tetrahydropyrazin-2-one
Click to Show/Hide
|
||||
| Structure |
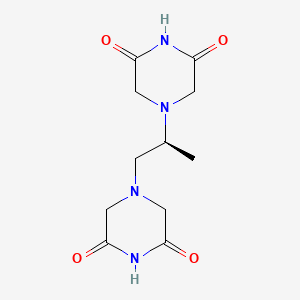 |
||||
| Formula |
C11H16N4O4
|
||||
| IUPAC Name |
4-[(2S)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione
|
||||
| Canonical SMILES |
CC(CN1CC(=O)NC(=O)C1)N2CC(=O)NC(=O)C2
|
||||
| InChI |
InChI=1S/C11H16N4O4/c1-7(15-5-10(18)13-11(19)6-15)2-14-3-8(16)12-9(17)4-14/h7H,2-6H2,1H3,(H,12,16,17)(H,13,18,19)/t7-/m0/s1
|
||||
| InChIKey |
BMKDZUISNHGIBY-ZETCQYMHSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | High mobility group protein B1 (HMGB1) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Wistar rats (250-300 g,n = 230) were purchased from Vitalriver. Ten rats were recruited in each group in the experiment. The rats in the control, DOX, FER-1 + DOX, NEC-1 + DOX, 3-MA + DOX, and Emricasan + DOX groups were used for the overall survival analysis, and those from control and DOX groups were used for detection of the expression of PTGS2 in the indicated organs of rats.
Click to Show/Hide
|
||||
| Response regulation | Dexrazoxane (DXZ) reduces cytotoxicity caused by Doxorubicin (DOX). HMGB1 was induced by DOX but was inhibited by DXZ or FER-1. Overexpression of HMGB1 promoted the ferroptosis and cardiotoxicity induced by DOX in the rats although silencing of HMGB1 showed opposite effects. | ||||
