Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0129)
| Name |
Carmustine
|
||||
|---|---|---|---|---|---|
| Synonyms |
carmustine; 154-93-8; 1,3-Bis(2-chloroethyl)-1-nitrosourea; BCNU; Gliadel; Carmubris; Carmustin; Nitrumon; BiCNU; Becenun; Bischloroethyl nitrosourea; Bi CNU; Carmustinum; 1,3-Bis(2-chloroethyl)nitrosourea; Bischlorethylnitrosurea; N,N'-BIS(2-CHLOROETHYL)-N-NITROSOUREA; Bischlorethylnitrosourea; Carmustina; Urea, N,N'-bis(2-chloroethyl)-N-nitroso-; NSC-409962; Bis(2-chloroethyl)nitrosourea; Gliadel Wafer; FDA 0345; SRI 1720; BiCNU (TN); DTI 015; NCI-C04773; SK 27702; C5H9Cl2N3O2; Carmustine (98%); Bis(chloroethyl)nitrosourea; NSC 409962; bis-chloroethylnitrosourea; NSC409962; CHEMBL513; 1,3-bis(2-chloroethyl)-1-nitroso-urea; CHEBI:3423; Urea, 1,3-bis(2-chloroethyl)-1-nitroso-; Becenum; DTXSID8022743; DTI-015; U68WG3173Y; FDA-0345; SRI-1720; 1,3-bis(2-chloroethyl)-3-nitrosourea; NCGC00015204-05; SK-27702; 1,3-Bis(.beta.-chloroethyl)-1-nitrosourea; Bischloroethylnitrosourea; Carmustinum [INN-Latin]; Carmustina [INN-Spanish]; DTXCID002743; Bis(2-chloroethyl)1-nitrosourea; Bis-N,N'-(chloroethyl)nitrosourea; CAS-154-93-8; CCRIS 810; 1,3-Bis(beta-chloroethyl)-1-nitrosourea; BCNU [Chloroethyl nitrosoureas]; SR-01000075736; EINECS 205-838-2; BRN 2049744; Camustine; Carustine; UNII-U68WG3173Y; AI3-52216; HSDB 7761; BiCNU; Nitrumon; Carmustine [USAN:USP:INN:BAN]; Carmustine- Bio-X; Prestwick_997; Gliadel (TN); Bischloroethyl nitrosourea [Chloroethyl nitrosoureas]; MFCD00057706; Gliadel (MGI Pharm); Spectrum_000265; Carmustine, >=98%; CARMUSTINE [MI]; CARMUSTINE [INN]; CARMUSTINE [JAN]; Spectrum4_000888; Spectrum5_000920; CAMUSTINE [VANDF]; CARMUSTINE [HSDB]; CARMUSTINE [USAN]; Lopac-C-0400; WLN: ONN2GVM2G; C 0400; CARMUSTINE [MART.]; SCHEMBL4503; CARMUSTINE [USP-RS]; CARMUSTINE [WHO-DD]; Lopac0_000188; Carmustine (JAN/USP/INN); KBioGR_001296; KBioSS_000745; MLS001333962; DivK1c_000835; AMY382; GTPL6800; CARMUSTINE [EP IMPURITY]; CARMUSTINE [ORANGE BOOK]; HMS502J17; KBio1_000835; KBio2_000745; KBio2_003313; KBio2_005881; CARMUSTINE [EP MONOGRAPH]; NINDS_000835; CARMUSTINE [USP MONOGRAPH]; HMS2092J22; HMS2230I05; HMS3260F17; HMS3369D17; Pharmakon1600-01503110; BCP27690; Tox21_110097; Tox21_500188; BDBM50015950; CCG-39925; NSC758392; STK624770; AKOS005558013; Tox21_110097_1; BCP9000490; DB00262; LP00188; NSC-758392; SDCCGSBI-0050176.P004; IDI1_000835; Urea,3-bis(2-chloroethyl)-1-nitroso-; BISCHLOROETHYL NITROSOUREA [IARC]; NCGC00015204-01; NCGC00015204-02; NCGC00015204-03; NCGC00015204-04; NCGC00015204-06; NCGC00015204-07; NCGC00015204-08; NCGC00015204-09; NCGC00015204-18; NCGC00093665-01; NCGC00093665-02; NCGC00093665-03; NCGC00093665-04; NCGC00260873-01; Urea,N'-bis(2-chloroethyl)-N-nitroso-; AC-24196; AS-12106; BC164289; HY-13585; N,N''-Bis(2-chloroethyl)-N-nitrosourea; NCI60_003931; SMR000058426; SBI-0050176.P003; C2634; EU-0100188; FT-0602937; S3669; BCNU; 1,3-Bis(2-chloroethyl)-1-nitrosourea; C06873; D00254; EN300-123541; AB00052431-07; AB00052431_08; A809590; Q415869; SR-01000075736-1; SR-01000075736-3; W-108025; Z1269141329; Carmustine, United States Pharmacopeia (USP) Reference Standard; 1-(2-Chloroethyl)-1-([(2-chloroethyl)amino]carbonyl)-2-oxohydrazine #
Click to Show/Hide
|
||||
| Structure |
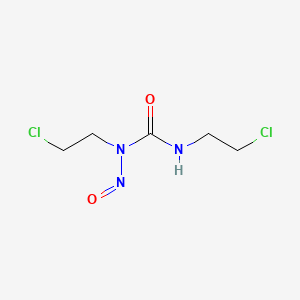 |
||||
| Formula |
C5H9Cl2N3O2
|
||||
| IUPAC Name |
1,3-bis(2-chloroethyl)-1-nitrosourea
|
||||
| Canonical SMILES |
C(CCl)NC(=O)N(CCCl)N=O
|
||||
| InChI |
InChI=1S/C5H9Cl2N3O2/c6-1-3-8-5(11)10(9-12)4-2-7/h1-4H2,(H,8,11)
|
||||
| InChIKey |
DLGOEMSEDOSKAD-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12 | |||
| Responsed Regulator | Glutathione reductase, mitochondrial (GSR) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| In Vivo Model |
Age-matched male BALB/c nude mice (4-6 weeks old) were used for the orthotopic mouse model. Cohorts of mice were randomized into different treatment groups. 4 x 106 tumor cells were suspended in a 50 ul PBS/Matrigel (356234, BD Biosciences) mixture (1:1 (v/v) ratio) for each group of mice and injected into the left liver lobes by surgical implantation.
Click to Show/Hide
|
||||
| Response regulation | The combination of sorafenib and carmustine (BCNU), a selective inhibitor of GSR, remarkably hamper tumor growth by enhancing ferroptotic cell death in vivo. SLC27A5 serves as a suppressor in sorafenib resistance and promotes sorafenib-triggered ferroptosis via restraining the NRF2/GSR pathway in hepatocellular carcinoma, providing a potential therapeutic strategy for overcoming sorafenib resistance. | ||||
