Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0094)
| Name |
Deferiprone
|
||||
|---|---|---|---|---|---|
| Synonyms |
deferiprone; 30652-11-0; 3-Hydroxy-1,2-dimethyl-4(1H)-pyridone; Ferriprox; 3-hydroxy-1,2-dimethylpyridin-4(1H)-one; CP20; 1,2-dimethyl-3-hydroxy-4-pyridone; 1,2-Dimethyl-3-hydroxypyrid-4-one; APO-66; 3-hydroxy-1,2-dimethylpyridin-4-one; 1,2-Dimethyl-3-hydroxypyridine-4-one; 4(1H)-Pyridinone, 3-hydroxy-1,2-dimethyl-; APO-066; DN-180-01-AF; Deferipron; MFCD00134497; Deferiprone (INN); PL-1; L1; CHEBI:68554; 1,2-Dimethyl-3-hydroxy-4(1H)-pyridinone; CP-20; DN-18001AF; NSC-758880; 2BTY8KH53L; MLS000069481; HK-1; PL1; DTXSID6040666; 4(1H)-Pyridinone, 1,2-dimethyl-3-hydroxy-; SMR000059136; DEFERIPRONE [INN]; L-1; CP20 (Chelating agent); Ferriprox (TN); UNII-2BTY8KH53L; BRN 1447108; Deferidone; Deferiprona; Deferum; Deferiprone [USAN:INN:BAN]; CCRIS 8318; 1,2-dimethyl-3-hydroxy-4-pyridinone; 3-Hydroxy-1,2-dimethyl-4-pyridinone; Opera_ID_366; 3-hydroxy-1,2-dimethyl-4(1H)-pyridinone; DEFERIPRONE [MI]; 4(1H)-Pyridone, 3-hydroxy-1,2-dimethyl-; Deferiprone (USAN/INN); DEFERIPRONE [USAN]; DEFERIPRONE [VANDF]; DEFERIPRONE [MART.]; SCHEMBL94474; DEFERIPRONE [WHO-DD]; MLS000758227; MLS001424029; MLS006011689; CHEMBL70927; DEFERIPRONE [EMA EPAR]; CRMD-001; GTPL7456; DTXCID4020666; HSDB 8335; TZXKOCQBRNJULO-UHFFFAOYSA-; DEFERIPRONE [ORANGE BOOK]; EX-A972; TZXKOCQBRNJULO-UHFFFAOYSA-N; CP020; DEFERIPRONE [EP MONOGRAPH]; HMS2051C05; HMS2232E24; HMS3264P17; HMS3393C05; HMS3873L13; Pharmakon1600-01504512; BCP09606; HY-B0568; 3-hydroxy-1,2-dimethylpyrid-4-one; Tox21_112429; BBL036482; BDBM50525976; CGP-37391; NSC758880; s4067; STL559018; 1,2-dimetyl-3-hydroxy-4-pyridinone; AKOS015902286; CCG-100760; CS-5240; DB08826; DS-5493; NC00010; NSC 758880; 3-hydroxy-1,2-dimethyl-4- pyridinone; 3-hydroxy-1,2-dimethyl-pyridin-4-one; NCGC00090548-01; NCGC00090548-02; NCGC00090548-04; NCGC00090548-05; AC-33026; BP-12415; SY052576; SBI-0207041.P001; CAS-30652-11-0; FT-0606424; H0944; D07416; 3-Hydroxy-1,2-dimethyl-4(1H)-pyridone, 98%; AB00572605_11; AB00572605_12; EN300-1718313; 3-hydroxy-1,2-dimethyl-1,4-dihydropyridin-4-one; A820470; Q749664; SR-01000721891; Deferiprone 1,2-DIMETHYL-3-HYDROXY-4-PYRIDONE; SR-01000721891-4; Z1255390865; InChI=1/C7H9NO2/c1-5-7(10)6(9)3-4-8(5)2/h3-4,10H,1-2H3
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
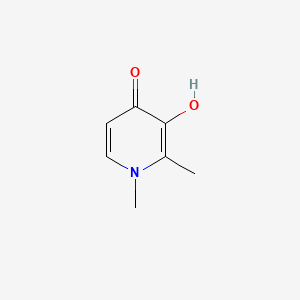 |
||||
| Formula |
C7H9NO2
|
||||
| IUPAC Name |
3-hydroxy-1,2-dimethylpyridin-4-one
|
||||
| Canonical SMILES |
CC1=C(C(=O)C=CN1C)O
|
||||
| InChI |
InChI=1S/C7H9NO2/c1-5-7(10)6(9)3-4-8(5)2/h3-4,10H,1-2H3
|
||||
| InChIKey |
TZXKOCQBRNJULO-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Nervous system disease | ICD-11: 8E7Z | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHNs (Rat hippocampal neuronal cells) | ||||
| In Vivo Model |
Sprague-Dawley rat pups at postnatal day (PND) 6 and 15-month-old male C57BL/6 mice were used in the present study. For ketamine GA, rat pups at PND 6 or 15-month-old mice received ketamine (75 mg/kg) intraperitoneally daily for three consecutive days. For sevoflurane GA, animals were put in an anaesthetizing chamber delivered with 3% sevoflurane plus 30% oxygen (O2) for 2 h daily for three consecutive days. For control experiments, 30% O2 was delivered at the same flow rate. For drug treatment, DFP (75 mg/kg, intraperitoneally, synthesized in China Peptides Co., Ltd., Shanghai, China) or DMT1i (50 mg/kg, orally, MedChemExpress, China) was administered to the animals 1 h before GA daily for three consecutive days.
Click to Show/Hide
|
||||
| Response regulation | Iron is essential for normal neuronal function, and excess iron in the brain is implicated in several neurodegenerative diseases. Chelating neurotoxic iron with deferiprone ameliorated general anaesthesia (GA)-induced cognitive deficits and suggest that iron restriction might provide a preventative effect for paediatric patients undertaking GA. | ||||
