Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0055)
| Name |
Zalcitabine
|
||||
|---|---|---|---|---|---|
| Synonyms |
zalcitabine; Dideoxycytidine; 7481-89-2; 2',3'-DIDEOXYCYTIDINE; ddCyd; HIVID; Cytidine, 2',3'-dideoxy-; ddC; 4-Amino-1-((2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one; Zalcitibine; NSC 606170; Ro 24-2027/000; Ro-24-2027/000; NSC-606170; CHEMBL853; 6L3XT8CB3I; 4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one; DTXSID0023747; CHEBI:10101; 1-(2,3-Dideoxy-beta-D-ribofuranosyl)cytosine; NCGC00090705-08; Ro-242027000; 4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one; Ro-24-2027000; 4-AMINO-1-[(2R,5S)-5-(HYDROXYMETHYL)OXOLAN-2-YL]-1,2-DIHYDROPYRIMIDIN-2-ONE; 2,3-dideoxycytidine; DTXCID703747; NSC606170; 4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2-one; SMR000058253; CCRIS 692; Hivid(TM); Hivid (TN); HSDB 7156; Interferon AD + ddC; ddC & GM-CSF; ddC & sCD4; PC-SOD & ddC; DDC (DDC); UNII-6L3XT8CB3I; BRN 0654956; Zalcitabine (JAN/USP/INN); DS-4152 & ddC; ddC & NP (from PHCA or HSA); SRI-7707; Zalcitabine [USAN:USP:INN:BAN]; 2',3'-Dideoxycytidine;ddC;Dideoxycytidine; CAS-7481-89-2; Zalcitabine- Bio-X; MFCD00012188; KS-1130; ddC & IFN.alpha.; .beta.-D-DDC; dideoxycytidine (DDC); ZALCITABINE [MI]; Prestwick0_001037; Prestwick1_001037; Prestwick2_001037; Prestwick3_001037; ddC & Interferon.alpha.; ZALCITABINE [INN]; ZALCITABINE [JAN]; 2', 3'-dideoxycytidine; ZALCITABINE [HSDB]; ZALCITABINE [IARC]; ZALCITABINE [USAN]; bmse000712; UPCMLD-DP115; D 5782; ZALCITABINE [VANDF]; SCHEMBL3598; TimTec1_004969; ZALCITABINE [MART.]; Lopac0_000360; BSPBio_001253; ZALCITABINE [USP-RS]; ZALCITABINE [WHO-DD]; 3'-Azido-3'-deoxythymidine/2',3'-Dideoxycytidine; 5-25-14-00313 (Beilstein Handbook Reference); MLS000069636; MLS000759540; MLS001055363; MLS001424210; MLS006011951; SPBio_003104; BPBio1_001378; GTPL4828; UPCMLD-DP115:001; 2',3'-Dideoxycytidine & sCD4(soluble recombinant protein); Zalcitabine (dideoxycytidine,ddc); ZALCITABINE [ORANGE BOOK]; HMS1548B19; HMS1571O15; HMS2051H18; HMS2090C12; HMS2098O15; HMS2236N08; HMS3261G21; HMS3715O15; Pharmakon1600-01502360; ZALCITABINE [USP IMPURITY]; .beta.-D-2',3'-Dideoxycytidine; Zalcitabine, 2'3'-Dideoxycytidine; BCP13878; Tox21_113491; Tox21_201655; Tox21_303169; Tox21_500360; AC-824; BDBM50145605; Lecithinized superoxide dismutase & .beta.-D-2',3'-Dideoxycytidine; NSC759655; s1719; AKOS015854844; AKOS015894505; Tox21_113491_1; CCG-101050; CS-1110; DB00943; LP00360; NC00300; NSC-759655; SDCCGSBI-0050348.P002; SRI-7707-13; SRI-7707-14; SRI-7707_15; SRI-7707_17; NCGC00090705-01; NCGC00090705-02; NCGC00090705-03; NCGC00090705-05; NCGC00090705-06; NCGC00090705-07; NCGC00090705-09; NCGC00090705-10; NCGC00090705-11; NCGC00090705-13; NCGC00090705-15; NCGC00090705-24; NCGC00090705-25; NCGC00179242-01; NCGC00257202-01; NCGC00259204-01; NCGC00261045-01; BD164564; BP-58631; HY-17392; ddC;Dideoxycytidine;2',3'-Dideoxycytidine; 2',3'-Dideoxycytidine & Interferon.alpha.; 2',3'-Dideoxycytidine, >=98% (HPLC); D3581; EU-0100360; SW197364-4; 2',3'-Dideoxycytidine, >=99.0% (HPLC); C07207; C76390; D00412; EN300-120633; Cytidine, 2',3'-dideoxy- & Interferon.alpha.; A838234; SR-01000075822; SR-01000736919; ZALCITABINE (DIDEOXYCYTIDINE,DDC) [VANDF]; J-700276; Q-201941; Q2344582; SR-01000075822-1; SR-01000736919-5; Cytidine, 2',3'-dideoxy- & Colony-stimulating factor; Ro-242027000/Ro-24-2027-000; Z1550648753; Zalcitabine, United States Pharmacopeia (USP) Reference Standard; 4-Amino-1-(5-hydroxymethyl-tetrahydro-furan-2-yl)-1H-pyrimidin-2-one; .beta.-D-2',3'-Dideoxycytidine & Granulocyte-macrophage colony-stimulating factor
Click to Show/Hide
|
||||
| Structure |
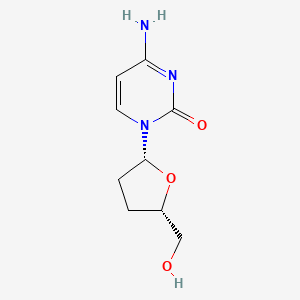 |
||||
| Formula |
C9H13N3O3
|
||||
| IUPAC Name |
4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one
|
||||
| Canonical SMILES |
C1CC(OC1CO)N2C=CC(=NC2=O)N
|
||||
| InChI |
InChI=1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1
|
||||
| InChIKey |
WREGKURFCTUGRC-POYBYMJQSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Pancreatic cancer | ICD-11: 2C10 | |||
| Responsed Regulator | Stimulator of interferon genes protein (STING1) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | ||||
| Cytosolic DNA-sensing pathway | hsa04623 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Capan-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0026 | ||
| In Vivo Model |
NOD SCID mice (394) were purchased from Charles River Laboratories. Indicated wild-type or gene knockdown PANC1 cells (5 x 106 cells) were subcutaneously injected into the dorsal side of NOD SCID mice. At day 7, these mice were administrated with the indicated drug (zalcitabine [50 mg/kg, per day by i.p.], H-151 [750 nM per mouse, once every other day by i.p.], chloroquine [50 mg/kg, once every other day by i.p.], or liproxstatin-1 [10 mg/kg, once every other day by i.p.]) for 2 weeks.
Click to Show/Hide
|
||||
| Response regulation | The antiviral drug zalcitabine can suppress pancreatic cancer cell growth through the induction of autophagy-dependent ferroptotic deathin vitroandin vivo. Mechanistically, these effects are dependent on mtDNA stress-induced activation of the CGAS-STING1 pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Pancreatic cancer | ICD-11: 2C10 | |||
| Responsed Regulator | Cyclic GMP-AMP synthase (CGAS) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | ||||
| Cytosolic DNA-sensing pathway | hsa04623 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Capan-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0026 | ||
| In Vivo Model |
NOD SCID mice (394) were purchased from Charles River Laboratories. Indicated wild-type or gene knockdown PANC1 cells (5 x 106 cells) were subcutaneously injected into the dorsal side of NOD SCID mice. At day 7, these mice were administrated with the indicated drug (zalcitabine [50 mg/kg, per day by i.p.], H-151 [750 nM per mouse, once every other day by i.p.], chloroquine [50 mg/kg, once every other day by i.p.], or liproxstatin-1 [10 mg/kg, once every other day by i.p.]) for 2 weeks.
Click to Show/Hide
|
||||
| Response regulation | The antiviral drug zalcitabine can suppress pancreatic cancer cell growth through the induction of autophagy-dependent ferroptotic deathin vitroandin vivo. Mechanistically, these effects are dependent on mtDNA stress-induced activation of the CGAS-STING1 pathway. | ||||
