Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0040)
| Name |
5-Azacitidine
|
||||
|---|---|---|---|---|---|
| Synonyms |
5-azacytidine; Azacitidine; 320-67-2; Ladakamycin; Azacytidine; Vidaza; Mylosar; 5-azacitidine; Azacitidinum; Azacitidina; Azacitidinum [INN-Latin]; 5-AZAC; Azacitidina [INN-Spanish]; C8H12N4O5; NSC-102816; U-18496; Onureg; 4-Amino-1-beta-D-ribofuranosyl-s-triazin-2(1H)-one; NSC102816; 5AzaC; 4-Amino-1-beta-d-ribofuranosyl-1,3,5-triazin-2(1H)-one; Antibiotic U 18496; 5-AZCR; 4-amino-1-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1,3,5-triazin-2(1H)-one; 5 AZC; 5-AC; NSC 102816; CHEBI:2038; M801H13NRU; DTXSID9020116; 4-Amino-1-(beta-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one; U-18,496; 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2-dihydro-1,3,5-triazin-2-one; WR-183027; NCGC00090851-04; 4-Amino-1-beta-D-ribofuranosyl-1,3,5-triazine-2(1H)-one; U 18496; 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2-one; DTXCID10116; 1,3,5-Triazin-2(1H)-one, 4-amino-1-.beta.-D-ribofuranosyl-; MFCD00006539; CCRIS 60; SMR000857239; Vidaza (TN); HSDB 6879; 5-aza-CR; SR-01000075662; EINECS 206-280-2; Azacitidine;5-AzaC;Ladakamycin; BRN 0620461; UNII-M801H13NRU; Azacitidine (JAN/USAN/INN); Azacitidine [USAN:INN:BAN]; 4-Amino-1-beta-D-ribofuranosyl-1,3,5-traizin-2(1H)-one; NS-17; 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,3,5-triazin-2-one; CAS-320-67-2; Azacitidine (Vidaza); 2-(beta-D-Ribofuranosyl)-4-amino-1,3,5-triazin-2-one; Antibiotic U18496; U18496; Spectrum_001262; AZACITIDINE [MI]; Spectrum2_000786; Spectrum3_001509; Spectrum4_000922; Spectrum5_001166; AZACITIDINE [INN]; AZACITIDINE [JAN]; AZACITIDINE [HSDB]; AZACITIDINE [IARC]; AZACITIDINE [USAN]; MolMap_000062; 4-Amino-1-.beta.-D-ribofuranosyl-s-triazin-2(1H)-one; A 2385; AZACITIDINE [VANDF]; SCHEMBL3741; AZACITIDINE [MART.]; CHEMBL1489; Azacitidine (5-Azacytidine); Lopac0_000035; AZACITIDINE [USP-RS]; AZACITIDINE [WHO-DD]; BSPBio_003157; KBioGR_001444; KBioGR_002556; KBioSS_001742; KBioSS_002565; MLS001333121; MLS001333122; MLS002153249; MLS002548894; DivK1c_000125; SPECTRUM1502111; SPBio_000892; AZACITIDINE [EMA EPAR]; GTPL6796; s-Triazin-2(1H)-one, 4-amino-1-beta-D-ribofuranosyl-; BCBcMAP01_000083; HMS500G07; KBio1_000125; KBio2_001742; KBio2_002556; KBio2_004310; KBio2_005124; KBio2_006878; KBio2_007692; KBio3_002657; KBio3_003034; NMUSYJAQQFHJEW-KVTDHHQDSA-; AZACITIDINE [ORANGE BOOK]; pyrimidine antimetabolite: inhibits nucleic acid replication; cMAP_000082; NINDS_000125; NMUSYJAQQFHJEW-KVTDHHQDSA-N; HMS1921J22; HMS2092D08; HMS2231F15; HMS3259D19; HMS3260G11; Pharmakon1600-01502111; 5-Azacytidine, >=98% (HPLC); Tox21_111032; Tox21_302985; Tox21_500035; BDBM50424715; CCG-39046; HB1374; NSC758186; s1782; Onureg (CC-486; oral azacitidine); AKOS015896938; Tox21_111032_1; AM83944; CS-1287; DB00928; LP00035; NC00672; NSC-758186; NSC103-627; 4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triaz; IDI1_000125; NCGC00090851-01; NCGC00090851-02; NCGC00090851-03; NCGC00090851-05; NCGC00090851-06; NCGC00090851-07; NCGC00090851-08; NCGC00090851-10; NCGC00090851-14; NCGC00090851-22; NCGC00178234-01; NCGC00256541-01; NCGC00260720-01; AS-13697; HY-10586; SRI-10756_10; SRI-10756_12; WR183027; SL-000003; EU-0100035; D03021; EN300-118700; F10504; A821115; Q416451; J-700085; SR-01000075662-1; SR-01000075662-3; SR-01000075662-7; BRD-K03406345-001-02-1; BRD-K03406345-001-27-8; 4-Amino-1-?-D-ribofuranosyl-1,3,5-triazin-2(1H)-one; Z1515383340; 4-Amino-1-(bet.-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one; 4-Amino-1-beta-D-ribofuranosyl-1,3,5-tr iazin-2(1H)-one; Azacitidine, United States Pharmacopeia (USP) Reference Standard; 4-Amino-1-(beta-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one; Ladakamycin; Azacitidine, Pharmaceutical Secondary Standard; Certified Reference Material; 1401238-97-8; 5-Azacytidine, Hybri-Max(TM), gamma-irradiated, lyophilized powder, BioXtra, suitable for hybridoma; 5AE; 6-amino-3-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1H-1,3,5-triazin-3-ium-2-one; InChI=1/C8H12N4O5/c9-7-10-2-12(8(16)11-7)6-5(15)4(14)3(1-13)17-6/h2-6,13-15H,1H2,(H2,9,11,16)/t3-,4-,5-,6-/m1/s1
Click to Show/Hide
|
||||
| Structure |
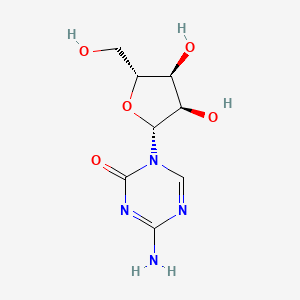 |
||||
| Formula |
C8H12N4O5
|
||||
| IUPAC Name |
4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2-one
|
||||
| Canonical SMILES |
C1=NC(=NC(=O)N1C2C(C(C(O2)CO)O)O)N
|
||||
| InChI |
InChI=1S/C8H12N4O5/c9-7-10-2-12(8(16)11-7)6-5(15)4(14)3(1-13)17-6/h2-6,13-15H,1H2,(H2,9,11,16)/t3-,4-,5-,6-/m1/s1
|
||||
| InChIKey |
NMUSYJAQQFHJEW-KVTDHHQDSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Head neck squamous cell carcinoma | ICD-11: 2D60 | |||
| Responsed Regulator | Cadherin-1 (CDH1) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell adhesion molecules | hsa04514 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AMC-HN-3 cells | Laryngeal squamous cell carcinoma | Homo sapiens | CVCL_5961 | |
| HN4 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_IS30 | ||
| HN5 cells | Squamous cell carcinoma | Homo sapiens | CVCL_8128 | ||
| HN6 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_8129 | ||
| NH-9 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_8132 | ||
| HN-10 cells | Laryngeal squamous cell carcinoma | Homo sapiens | CVCL_8124 | ||
| In Vivo Model |
Six-week-old athymic BALB/c male nude mice (nu/nu) were purchased from OrientBio (Seoul, Republic of Korea). HN9 cells with transfection of CDH1 or control vector or HN4 cells with ZEB1 or control vector were subcutaneously injected into the bilateral flank of nude mice. From the day when gross nodules were detected in tumor implants, mice were subjected to different treatments: vehicle or sulfasalazine (250 mg/kg daily per intraperitoneal route). Each group included six mice.
Click to Show/Hide
|
||||
| Response regulation | Histone deacetylase SIRT1 gene silencing or pharmacological inhibition by EX-527 suppressed EMT and consequently decreased ferroptosis, whereas SIRT inducers, resveratrol and SRT1720, increased ferroptosis. In head and neck cancer (HNC) cells with low expression of E-cadherin, the treatment of 5-azacitidine diminished the hypermethylation of CDH1, resulting in increased E-cadherin expression and decreased ferroptosis susceptibility. | ||||
