Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0027)
| Name |
Salinomycin
|
||||
|---|---|---|---|---|---|
| Synonyms |
salinomycin; Procoxacin; 53003-10-4; Coxistac; Bio-cox; CHEBI:80025; 62UXS86T64; Salinomycin (INN); SALINOMYCIN [INN]; Salinomicina; Salinomycine; Salinomycinum; Salinomycin [INN:BAN]; Salinomycine [INN-French]; Salinomycinum [INN-Latin]; Salinomicina [INN-Spanish]; UNII-62UXS86T64; K 364; HSDB 7032; Procoxacin (TN); AHR 3096; EINECS 258-290-1; SALINOMYCIN [MI]; SALINOMYCIN [JAN]; SALINOMYCIN [HSDB]; SCHEMBL36890; CHEMBL1208572; DTXSID4048486; GTPL11088; KQXDHUJYNAXLNZ-XQSDOZFQSA-N; BDBM430647; NSC757437; s2352; s8129; Salinomycin (from Streptomyces albus); CCG-208535; CS-1299; DB11544; NSC-757437; BS-17023; E716; HY-15597; D08502; A829344; Q411909; J-524236; SR-05000002207-3; Salinomycin, from Streptomyces albus, >=98% (HPLC); (2R)-2-[(2R,5S,6R)-6-[(2S,3S,4S,6R)-6-[(2S,5S,7R,9S,10S,12R,15R)-2-[(2R,5R,6S)-5-ethyl-5-hydroxy-6-methyloxan-2-yl]-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro[4.1.5?.3?]pentadec-13-en-9-yl]-3-hydroxy-4-methyl-5-oxooctan-2-yl]-5-methyloxan-2-yl]butanoic acid; (2R)-2-[(2R,5S,6R)-6-[(2S,3S,4S,6R)-6-[(3S,5S,7R,9S,10S,12R,15R)-3-[(2R,5R,6S)-5-ethyl-5-hydroxy-6-methyloxan-2-yl]-15-hydroxy-3,10,12-trimethyl-4,6,8-trioxadispiro[4.1.57.35]pentadec-13-en-9-yl]-3-hydroxy-4-methyl-5-oxooctan-2-yl]-5-methyloxan-2-yl]butanoic acid; (R)-2-((2R,5S,6R)-6-((2S,3S,4S,6R)-6-((2S,5S,7R,9S,10S,12R,15R)-2-((2R,5R,6S)-5-ethyl-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro[4.1.57.35]pentadec-13-en-9-yl)-3-hydroxy-4-methyl-5-oxooctan-2-yl)-5-methyltetrahydro-2H-pyran-2-yl)butanoic acid; (R)-2-((2R,5S,6R)-6-((2S,3S,4S,6R)-6-((2S,5S,7R,9S,10S,12R,15R)-2-((2R,5R,6S)-5-ethyl-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro[4.1.57.35]pentadec-13-en-9-yl)-3-hydroxy-4-methyl-5-oxooctan-2-yl)-5-methyltetrahydro-2H-pyran-2-yl)butanoicacid; sodium 2-[6-[5-[3-(5-ethyl-5-hydroxy-6-methyl-tetrahydropyran-2-yl)-15-hydroxy-3,10,12-trimethyl-4,6,8-trioxadispiro[4.1.5^{7}.3^{5}]pentadec-13-en-9-yl]-2-hydroxy-1,3-dimethyl-4-oxo-heptyl]-5-methyl-tetrahydropyran-2-yl]butanoate
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
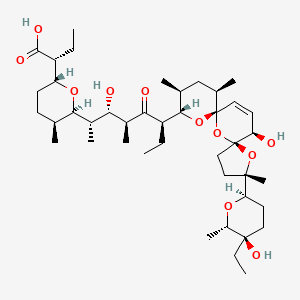 |
||||
|
3D MOL
|
|||||
| Formula |
C42H70O11
|
||||
| IUPAC Name |
(2R)-2-[(2R,5S,6R)-6-[(2S,3S,4S,6R)-6-[(3S,5S,7R,9S,10S,12R,15R)-3-[(2R,5R,6S)-5-ethyl-5-hydroxy-6-methyloxan-2-yl]-15-hydroxy-3,10,12-trimethyl-4,6,8-trioxadispiro[4.1.57.35]pentadec-13-en-9-yl]-3-hydroxy-4-methyl-5-oxooctan-2-yl]-5-methyloxan-2-yl]butanoic acid
|
||||
| Canonical SMILES |
CCC(C1CCC(C(O1)C(C)C(C(C)C(=O)C(CC)C2C(CC(C3(O2)C=CC(C4(O3)CCC(O4)(C)C5CCC(C(O5)C)(CC)O)O)C)C)O)C)C(=O)O
|
||||
| InChI |
InChI=1S/C42H70O11/c1-11-29(38(46)47)31-15-14-23(4)36(50-31)27(8)34(44)26(7)35(45)30(12-2)37-24(5)22-25(6)41(51-37)19-16-32(43)42(53-41)21-20-39(10,52-42)33-17-18-40(48,13-3)28(9)49-33/h16,19,23-34,36-37,43-44,48H,11-15,17-18,20-22H2,1-10H3,(H,46,47)/t23-,24-,25+,26-,27-,28-,29+,30-,31+,32+,33+,34+,36+,37-,39-,40+,41-,42-/m0/s1
|
||||
| InChIKey |
KQXDHUJYNAXLNZ-XQSDOZFQSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HMLER cells | Normal | Homo sapiens | CVCL_DG85 | |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| In Vivo Model |
MCF-7 cell cultures were collected, enzymatically dissociated, washed with PBS, and re-suspended in a PBS/Matrigel mixture (1:1 v/v). The mixture (0.1 mL) was then implanted in the mammary fat pad of 5-week-old female AthymicNude-Fox1nu mice bilaterally (Harlan, France). Mice received estradiol supplementation (0.4 mg/kg) the same day and 7 days from cell injection, and were observed and palpated for tumor appearance. Mice were treated with AM5 (1 mg/kg body weight/day) by means of intraperitoneal injections every 5 working days of the week.
Click to Show/Hide
|
||||
| Response regulation | A synthetic derivative of salinomycin, which we named ironomycin (AM5), exhibits a more potent and selective activity against breast cancer stem cells (CSCs) in vitro and in vivo, by accumulating and sequestering iron in lysosomes. | ||||
