Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0021)
| Name |
Estradiol
|
||||
|---|---|---|---|---|---|
| Synonyms |
estradiol; beta-Estradiol; 17beta-Estradiol; 50-28-2; Oestradiol; Dihydrofolliculin; Estrace; Vivelle; Ovocyclin; progynon; Dihydrotheelin; Dihydroxyestrin; Diogynets; Gynoestryl; Aquadiol; Climara; Diogyn; Gynergon; Vagifem; Dimenformon; Follicyclin; Oestroglandol; Aerodiol; Bardiol; Corpagen; Divigel; Estraderm; Estrovite; Femestral; Ginosedol; Lamdiol; Macrodiol; Oestergon; Ovasterol; Ovastevol; Perlatanol; Primofol; Profoliol; Syndiol; Altrad; Evorel; Dihydromenformon; Estraldine; Estroclim; Estrogel; Menorest; Nordicol; Ovahormon; Femogen; Systen; Zumenon; estradiol-17beta; cis-Estradiol; D-Oestradiol; D-Estradiol; Estraderm TTS; Progynon DH; Climaderm; Compudose; Dermestril; Ovocycline; Trocosone; Estring; Innofem; Oesclim; Alora; Encore; Dihydroxyoestrin; 17beta-Oestradiol; Progynon-DH; Estrasorb; Fempatch; Gynestrel; Gynodiol; Microdiol; Oestrogynal; Ovociclina; Ovocylin; Tradelia; Esclim; Macrol; Menest; Zerella; Estroclim 50; Oestradiol R; SK-Estrogens; Estring vaginal ring; Elestrin; Estrifam; Extrasorb; Ginedisc; Estreva; Evamist; Femtran; Trial SAT; Theelin, dihydro-; GynPolar; Compudose 200; Compudose 365; Sandrena Gel; Sisare Gel; Estrofem 2; Estradot; Menostar; Oestrogel; Zesteem; 17 beta-Estradiol; Climara Forte; Profoliol B; Estraderm TTS 50; Oestradiol Berco; Estraderm MX; 17b-Oestradiol; VIVELLE-DOT; NSC-9895; Estrapak 50; Estradiol-17-beta; Estradiolum; Estrodiolum; Gelestra; 3,17-Epidihydroxyestratriene; Dihydroxyesterin; cis-Oestradiol; Estrogens, esterified; 17.beta.-Estradiol; B-Estradiol; Epiestriol 50; 17beta oestradiol; Estra-1,3,5(10)-triene-3,17beta-diol; Estradiol Anhydrous; E(sub 2); 17-beta-estradiol; Estradiol-17 beta; Elestrim; Femanest; Femestrol; Minivelle; Yuvvexy; 3,17-Epidihydroxyoestratriene; Sandrena 1; 17b-Estradiol; Estraderm (TN); [3H]-estradiol; 17; A-estradiol; 17; A-Oestradiol; Estrogel (TN); Climara (TN); Divigel (TN); Estrace (TN); Estring (TN); Innofem (TN); Vagifem (TN); Vivelle (TN); (17beta)-Estra-1,3,5(10)-triene-3,17-diol; 17.beta.-Oestradiol; CCRIS 280; CHEBI:16469; HSDB 3589; 3,17.beta.-Estradiol; .beta.-Estradiol; (8R,9S,13S,14S,17S)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol; EINECS 200-023-8; .beta.-Oestradiol; [3H]]estradiol; NSC-20293; Estradiol 17-beta; 17-beta-oestradiol; UNII-4TI98Z838E; IMVEXXY; TX-004HR; Estradiol, .beta.-; Estradiol-17.beta.; Estra-1,3,5(10)-triene-3,17-diol (17beta)-; Oestradiol-17.beta.; 17-.beta.-oestradiol; 4TI98Z838E; ESTRADIOL HEMIHYDRATE; Oestra-1,3,5(10)-triene-3,17beta-diol; Estrasorb (TN); 17.beta.-OH-estradiol; 17.beta.-OH-oestradiol; 17beta estradiol (E2); 17beta-Estra-1,3,5(10)-triene-3,17-diol; WC3011; C18H24O2; CHEMBL135; (17beta)-estra-1(10),2,4-triene-3,17-diol; D-3,17.beta.-Estradiol; WC-3011; D-3,17.beta.-Oestradiol; Estra-1,3,5(10)-triene-3,17-diol, (17beta)-; MLS000069494; 3,17b-Dihydroxyestra-1,3,5(10)-triene; (+)-3,17.beta.-Estradiol; 141290-02-0; Oestradiolum; DTXSID0020573; Estradiol valerate metabolite e2; BIJUVA COMPONENT ESTRADIOL; NSC9895; ANGELIQ COMPONENT ESTRADIOL; EC 200-023-8; ORIAHNN COMPONENT ESTRADIOL; PREFEST COMPONENT ESTRADIOL; ACTIVELLA COMPONENT ESTRADIOL; ESTRADIOL COMPONENT OF BIJUVA; COMBIPATCH COMPONENT ESTRADIOL; [3H]E2; ESTRADIOL COMPONENT OF ANGELIQ; ESTRADIOL COMPONENT OF ORIAHNN; ESTRADIOL COMPONENT OF PREFEST; CLIMARA PRO COMPONENT ESTRADIOL; Oestradiol-17beta; (8R,9S,13S,14S,17S)-13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol; E2; ESTRADIOL COMPONENT OF ACTIVELLA; ESTRADIOL COMPONENT OF COMBIPATCH; Estradiolum [INN]; Estradiolo [DCIT]; NCGC00091544-04; Oestradiol-17-beta; ESTRADIOL COMPONENT OF CLIMARA PRO; Estradiolo; SMR000059126; Estradiol-3,17beta; 17-beta-OH-estradiol; 3,17-beta-Estradiol; 3,17beta-Dihydroxyestra-1,3,5(10)-triene; 3,17-beta-Oestradiol; D-3,17beta-Estradiol; 3,17.beta.-Dihydroxyestra-1,3,5(10)-triene; Methyl 1-cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylate.; Benzogynestry; Estropause; Estasorb; (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-5,14-diol; [2,4,6,7-3H]-E2; E 2; DTXCID80573; ESTRADIOL (USP MONOGRAPH); ESTRADIOL [USP MONOGRAPH]; Destradiol; ESTRADIOL IMPURITY A (IP); ESTRADIOL IMPURITY A [IP]; Zesteen; .alpha.-Estradiol; 17-beta-OH-oestradiol; 17?-Estradiol; D-3,17beta-Oestradiol; D-3,17-beta-Estradiol; Estrodiolum [INN-Latin]; Estra-1,3,5(10)-triene-3,17-diol (17.beta.)-; D-3,17-beta-Oestradiol; Activella (Salt/Mix); Combipatch (Salt/Mix); component of Menrium; Estradiol-17 alpha; Climara Pro (Salt/Mix); 3,17beta-Estradiol; 3,17-.beta.-Oestradiol; Estraderm TTS 100; ETHINYLESTRADIOL IMPURITY D (EP IMPURITY); ETHINYLESTRADIOL IMPURITY D [EP IMPURITY]; 3,3,5(10)-triene; SR-01000075866; 17-.BETA.-Estradiol; 1,3,5-Estratriene-3,17-beta-diol; oestrodiol; Estradiol [USP:INN:BAN]; 3,17beta-Dihydroxyestra-1,3,5-triene; Estradurin; 3,17beta-Dihydroxyoestra-1,3,5-triene; delta-Estradiol; dihydro-Theelin; 3,17-beta-Dihydroxyoestra-1,3,5-triene; NSC20293; beta -estradiol; delta-Oestradiol; Estra-1,3,5(10)-triene-3,17-beta-diol; Estrogel HBF; Oestra-1,3,5(10)-triene-3,17-beta-diol; 1jgl; 1lhu; 1qkt; 1qku; 3,17-beta-Dihydroxyestra-1,3,5(10)-triene; [3H]estradiol; 1,3,5-Estratriene-3,17.beta.-diol; 3,17-beta-Dihydroxy-1,3,5(10)-oestratriene; CAS-50-28-2; 17-beta-Estra-1,3,5(10)-triene-3,17-diol; 17beta-Oestra-1,3,5(10)-triene-3,17-diol; .alpha.-Oestradiol; 17-beta-Oestra-1,3,5(10)-triene-3,17-diol; Estra-1,3,5(10)-triene-3,17-diol; Prestwick_207; 3,17b-Estradiol; Bio-E-Gel; Fem7; [3H]-Estrogen; 17 ?-Estradiol; 3,17.beta.-Dihydroxyestra-1,3,5-triene; 3,17.beta.-Dihydroxyoestra-1,3,5-triene; CMC_11154; Therapeutic Estradiol; 1,3,5,(10)-Estratrien-3,17.beta.-diol; SL-1100; Estra-1,3,5(10)-triene-3,17.beta.-diol; Oestra-1,3,5(10)-triene-3,17.beta.-diol; 2j7x; 3,17.beta.-Dihydroxy-1,3,5(10)-estratriene; 3,17.beta.-Dihydroxy-1,3,5(10)-oestratriene; 3,3,5-triene; [3H]17beta-estradiol; ESTRADIOL [INN]; ESTRADIOL [JAN]; (17?)-Estra-1,3,5(10)-triene-3,17-diol; 17.beta.-Estra-1,3,5(10)-triene-3,17-diol; ESTRADIOL [MI]; ESTRADIOL [HSDB]; ESTRADIOL [INCI]; 17.beta.-Oestra-1,3,5(10)-triene-3,17-diol; Opera_ID_1688; Prestwick0_000441; Prestwick1_000441; Prestwick2_000441; Prestwick3_000441; Spectrum5_002055; 17beta-estradiol (E2); ESTRADIOL [VANDF]; 3,17beta-Dihydroxy-1,3,5(10)-estratriene; alpha-estradiol (obsolete); beta-Estradiol, >=98%; bmse000642; Epitope ID:136018; (+)-3,17b-Estradiol; E 8875; ESTRADIOL [USP-RS]; ESTRADIOL [WHO-DD]; SCHEMBL8049; (+)-3,17beta-Estradiol; Estradiol (JAN/USP/INN); Tritiated estradiol-17-beta; BIDD:PXR0065; Lopac0_000503; S-21400; BSPBio_000482; BSPBio_001065; KBioGR_000405; KBioGR_002269; KBioSS_000405; KBioSS_002270; MLS000758312; MLS001076331; MLS001424022; BIDD:ER0125; SPBio_002421; ESTRADIOL [GREEN BOOK]; BPBio1_000532; GTPL1012; GTPL1013; ESTRADIOL [ORANGE BOOK]; NIOSH/KG7030000; BDBM17292; KBio2_000405; KBio2_002269; KBio2_002973; KBio2_004837; KBio2_005541; KBio2_007405; KBio3_000769; KBio3_000770; KBio3_002749; 1a52; 1g50; 2d06; cMAP_000005; Bio1_000403; Bio1_000892; Bio1_001381; Bio2_000363; Bio2_000843; HMS1362E07; HMS1569I04; HMS1792E07; HMS1990E07; HMS2051C17; HMS2090E18; HMS2096I04; HMS2236H04; HMS3261F07; HMS3403E07; HMS3713I04; HMS3884A08; beta-Estradiol, analytical standard; (8S,9S,13S,14S,17S)-13-Methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol; BCP08579; ESTRADIOL 17-BETA [VANDF]; HY-B0141; Tox21_111148; Tox21_202057; Tox21_300288; Tox21_500503; 1,5-Estratriene-3,17.beta.-diol; HB2494; LMST02010001; s1709; Estradiol-17-beta-6,7-(sup 3)H; [2,4,6,7-3H]-17beta-estradiol; AKOS015896570; CCG-100808; CS-1938; DB00783; LP00503; NC00058; SDCCGSBI-0050487.P002; IDI1_002118; SMP1_000121; NCGC00091544-00; NCGC00091544-01; NCGC00091544-02; NCGC00091544-05; NCGC00091544-06; NCGC00091544-07; NCGC00091544-09; NCGC00091544-10; NCGC00091544-11; NCGC00091544-12; NCGC00091544-13; NCGC00091544-14; NCGC00091544-15; NCGC00091544-16; NCGC00091544-18; NCGC00091544-27; NCGC00179321-01; NCGC00179321-02; NCGC00254177-01; NCGC00259606-01; NCGC00261188-01; 17-E; AC-22346; AS-13729; CPD000059126; Estra-1,3,5(10)-triene-3,17b-diol; Oestra-1,3,5(10)-triene-3,17b-diol; WLN: L E5 B666TTT&J E1 FQ OQ; 17 beta-Estradiol, 1mg/ml in Acetonitrile; Estra-1,5(10)-triene-3,17.beta.-diol; Estradiol, meets USP testing specifications; 3,17beta-dihydroxyestra-1,3,5(10)-trien; EU-0100503; KG70300000; Oestra-1,5(10)-triene-3,17.beta.-diol; C00951; D00105; EN300-119518; (17b)-Estra-1,3,5(10)-triene-3,17-diol; 13b-Methyl-1,3,5(10)-gonatriene-3,17b-ol; 17.beta.-Estra-1,5(10)-triene-3,17-diol; 17.beta.-Oestra-1,5(10)-triene-3,17-diol; 17-beta-Estradiol 100 microg/mL in Acetonitrile; Q422416; SR-01000721892; 13beta-Methyl-1,3,5(10)-gonatriene-3,17beta-ol; Estra-1,5(10)-triene-3,17-diol (17.beta.)-; ESTRADIOL BENZOATE IMPURITY A [EP IMPURITY]; ESTRADIOL VALERATE IMPURITY A [EP IMPURITY]; Q-201503; SR-01000075866-1; SR-01000075866-4; SR-01000721892-3; BRD-K18910433-001-04-4; BRD-K18910433-001-43-2; estra-1(10),2,4-triene-3,17-diol, (17beta)-; Estra-1,5(10)-triene-3,17-diol, (17.beta.)-; Z1521553843; B8B5AEF5-4957-49EB-A14F-444A8212C482; beta-Estradiol, BioReagent, powder, suitable for cell culture; Estradiol, United States Pharmacopeia (USP) Reference Standard; 13.BETA.-METHYL-1,3,5(10)-GONATRIENE-3,17.BETA.-OL; beta-Estradiol, powder, gamma-irradiated, suitable for cell culture; Estra-1,3,5(10)-triene-3,17-diol, (6,7-(sup 3)H,17-beta)-; (9beta,13alpha,14beta,17alpha)-estra-1,3,5(10)-triene-3,17-diol; Estradiol, Pharmaceutical Secondary Standard; Certified Reference Material; (13S,17S)-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-beta-diol; (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0;{2,7}.0;{11,15}]heptadeca-2,4,6-triene-5,14-diol; (1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2,4,6-triene-5,14-diol
Click to Show/Hide
|
||||
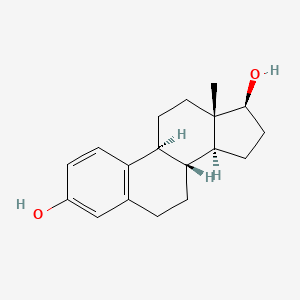
| Structure |
 |
||||
| Formula |
C18H24O2
|
||||
| IUPAC Name |
(8R,9S,13S,14S,17S)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol
|
||||
| Canonical SMILES |
CC12CCC3C(C1CCC2O)CCC4=C3C=CC(=C4)O
|
||||
| InChI |
InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1
|
||||
| InChIKey |
VOXZDWNPVJITMN-ZBRFXRBCSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Acute kidney failure | ICD-11: GB60 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| NRK-49F cells | Normal | Rattus norvegicus | CVCL_2144 | ||
| HK-2 cells | Normal | Homo sapiens | CVCL_0302 | ||
| C2C12 cells | Normal | Mus musculus | CVCL_0188 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| NRK-52E cells | Normal | Rattus norvegicus | CVCL_0468 | ||
| LLC-PK1 cells | Normal | Sus scrofa | CVCL_0391 | ||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| hUPECs (Human urine-derived podocyte-like epithelial cells) | |||||
| In Vivo Model |
C57BL/6N male mice (CLEA Japan), aged 8-9 weeks, were used. AKI was induced by intraperitoneal injection of cisplatin solution (16 or 17 mg/kg as indicated; Nichi-Iko Pharmaceutical). Mice were orally treated with water only, promethazine (20 mg/kg in water), or rifampicin (20 mg/kg in 0.5% methylcellulose) every 12 hours for 4 days starting 30 minutes before the cisplatin injection, or orally treated with promethazine (20 mg/kg) in the following groups: (1) no promethazine, (2) pretreatment 30 minutes before cisplatin injection, (3) treatment from 30 minutes before injection to 24 hours after injection, (4) treatment from 24 to 96 hours after injection, and (5) treatment every 12 hours from 30 minutes before injection to 96 hours after injection.
Click to Show/Hide
|
||||
| Response regulation | Eight drugs and hormones that showed antiferroptotic activity, including omeprazole, indole-3-carbinol, rifampicin, promethazine, carvedilol, propranolol, estradiol, and triiodothyronine. Moreover, in mice, the drugs ameliorated acute kidney injury and liver injury, with suppression of tissue lipid peroxidation and decreased cell death. | ||||
