Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0019)
| Name |
Testosterone
|
||||
|---|---|---|---|---|---|
| Synonyms |
testosterone; 58-22-0; Androderm; Testim; Testosteron; Mertestate; Testoderm; Virosterone; Sustanon; Homosterone; Androlin; Testiculosterone; AndroGel; Synandrol F; Testosteroid; Andronaq; Andropatch; Andrusol; Homosteron; Orquisteron; Perandren; Primotest; Primoteston; Sustanone; Testandrone; Testobase; Testogel; Testopropon; Testrone; Oreton; Relibra; Teslen; Testryl; Cristerona T; Testoviron T; Neotestis; Malerone; trans-Testosterone; Cristerone T; Testoviron Schering; Geno-cristaux gremy; Testostosterone; Testolin; Testoviron; LibiGel; 17beta-Hydroxyandrost-4-en-3-one; Sustason 250; Percutacrine androgenique; Neo-Hombreol F; Oreton-F; Testosteronum; Intrinsa; Testosterona; Striant; Testoject-50; Testoderm Tts; Testosterone hydrate; Neo-testis; Testro AQ; Malestrone (amps); Andro 100; Virormone; beta testosterone; Malogen, aquaspension injection; Vogelxo; Testosteronum [INN-Latin]; Testosterona [INN-Spanish]; Androst-4-en-17beta-ol-3-one; CDB 111C; Livensa; Nasobol; Natesto; Axiron; Tefina; CompleoTRT; Testopel Pellets; 17beta-Hydroxy-4-androsten-3-one; 7-beta-Hydroxyandrost-4-en-3-one; COL 1621; delta4-Androsten-17beta-ol-3-one; Testosterone ciii; 17beta-Hydroxyandrost-4-ene-3-one; 17-beta-Hydroxyandrost-4-en-3-one; 4-Androsten-17beta-ol-3-one; Androgel 1%; CP 601B; CCRIS 574; 17-Hydroxy-(17beta)-androst-4-en-3-one; Androgel 1.62%; 17beta-Hydroxy-delta(sup4)-androsten-3-one; delta(sup 4)-Androsten-17(beta)-ol-3-one; 17-Hydroxy-(17-beta)-androst-4-en-3-one; 17-beta-Hydroxy-delta(sup 4)-androsten-3-one; AA 2500; Androst-4-en-3-one, 17beta-hydroxy-; CHEBI:17347; HSDB 3398; Androst-4-en-3-one, 17-beta-hydroxy-; NSC 9700; EINECS 200-370-5; (+)-testosterone; UNII-3XMK78S47O; ANDROLAN; Androst-4-en-3-one, 17-hydroxy-, (17beta)-; 3XMK78S47O; DTXSID8022371; (17beta)-17-Hydroxyandrost-4-en-3-one; Oreton F; ANDROID-T; LPCN 1021; NSC-9700; Androst-4-en-3-one, 17-hydroxy-, (17-beta)-; DTXCID302371; (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one; Androst-4-en-3-one, 17-hydroxy-, (17b)-; EC 200-370-5; TESTOSTERONE (MART.); TESTOSTERONE [MART.]; Halotensin; Androsorb; Fortesta; Testaqua; Testopel; TESTOSTERONE (EP IMPURITY); TESTOSTERONE [EP IMPURITY]; TESTOSTERONE (EP MONOGRAPH); TESTOSTERONE [EP MONOGRAPH]; Androst-4-en-3-one, 17-hydroxy-, (17.beta.)-; TESTOSTERONE (USP MONOGRAPH); TESTOSTERONE [USP MONOGRAPH]; T-Cypionate; (8alpha,10alpha,13alpha,14beta,17alpha)-17-hydroxyandrost-4-en-3-one; Virilon IM; Testrin-P.A; Androderm (TN); 17.beta.-Testosterone; Androgel (TN); SMR000058344; Andronate 100; Andronate 200; Andropository 200; Striant (TN); Testamone 100; Bio-T-Gel; Testim (TN); Everone 200; Andryl 200; Scheinpharm Testone-Cyp; Testred Cypionate 200; Testosterone (JAN/USP); Androst-4-en-3-on-17B-ol; CHEMBL386630; component of Duogen (Salt/Mix); component of Tostrex (Salt/Mix); component of Di-Met (Salt/Mix); NSC9700; component of Intrinsa (Salt/Mix); Epitestosteron; Lumitestosteron; 4-Androsten-17.beta.-ol-3-one; 4-Androsten-3-one-17.beta.-ol; Tostrelle; Tostrex; Viatrel; component of Di-Genik (Salt/Mix); 4-Androsten-3-one, 17b-hydroxy-; Testosterone [USP:INN:BAN]; 9b-testosterone; component of Mal-O-Fem (Salt/Mix); Testostroval-PA; 4-androstene-17beta-ol-3-one; 17b-hydroxy-4-androsten-3-one; delta4-androsten-17b-ol-3-one; Testosterone, 1; 3kdm; 17b-Testosterone; 17-.beta.-Hydroxyandrost-4-en-3-one; NCGC00091018-01; 8-iso-testosterone; 17 beta Hydroxy 4 Androsten 3 one; 17-beta-Hydroxy-4-Androsten-3-one; (+-)-testosterone; Axiron (TN); CMC_13449; 13-iso-testosterone; mpp10; TESTSTERONE; 9b,10a-testosterone; 17.beta.-Hydroxy-.DELTA.4-androsten-3-one; 17-Hydroxyandrost-4-en-3-one, (17.beta.)-; Therapeutic Testosterone; (+-)-retrotestosterone; Testosterone, >=98%; TESTOSTERONE [MI]; TESTOSTERONE [INN]; TESTOSTERONE [JAN]; Epitope ID:135865; TESTOSTERONE [HSDB]; TESTOSTERONE [INCI]; Testosterone EP Impurity C; (+-)-8-iso-testosterone; SCHEMBL8452; TESTOSTERONE [VANDF]; 4-Androsten-3-one-17b-ol; MLS000563091; MLS001032098; MLS001306401; MLS001424262; MLS002174283; BIDD:ER0555; TESTOSTERONE [WHO-DD]; D4-Androsten-17b-ol-3-one; Androst-4-en-17b-ol-3-one; BDBM8885; GTPL2858; Androst-4-ene-17b-ol-3-one; TESTOSTERONE [EMA EPAR]; 17-hydroxy-D4-androsten-3-one; 17-hydroxy-androst-4-en-3-one; 17beta-hydroxy-4-androsten-3one; TESTOSTERONE [GREEN BOOK]; Testosterone, cell culture tested; 17b-Hydroxy-androst-4-en-3-on; 17b-Hydroxy-D4-androsten-3-one; 17b-Hydroxyandrost-4-ene-3-one; 1i37; Testosterone, 1mg/ml in Methanol; 17a-hydroxy-androst-4-en-3-one; 17b-hydroxy-androst-4-en-3-one; HMS2052N11; HMS2272B03; HMS2272P03; TESTOSTERONE [ORANGE BOOK]; (8R,9S,10R,13S,14S,17S)-17-Hydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3(2H)-one; 17-Hydroxy-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one; Tox21_200689; HY-15554A; LMST02020002; RB3046; 17b-hydroxy-8a-androst-4-en-3-one; rac-17b-hydroxy-androst-4-en-3-one; AKOS015894897; 17a-hydroxy-13a-androst-4-en-3-one; 17a-hydroxy-14b-androst-4-en-3-one; 17b-hydroxy-13a-androst-4-en-3-one; CCG-101189; CS-1415; DB00624; Human Serum - Testosterone, low level; NC00439; CAS-58-22-0; Human Serum - Testosterone, high level; Testosterone 1.0 mg/ml in Acetonitrile; (17?)-17-Hydroxyandrost-4-en-3-one; 17b-hydroxy-(8a)-androst-4-en-3-one; 17b-hydroxy-(9b)-androst-4-en-3-one; NCGC00258243-01; (17b)-17-hydroxy-androst-4-en-3-one; 17a-hydroxy-(13a)-androst-4-en-3-one; 17b-hydroxy-(10a)-androst-4-en-3-one; 17b-hydroxy-(13a)-androst-4-en-3-one; AC-14899; CPD000058344; SMR001261453; Testosterone, purum, >=99.0% (HPLC); Testosterone 100 microg/mL in Acetonitrile; rac-17b-hydroxy-(13a)androst-4-en-3-one; rac-17b-hydroxy-(8a)-androst-4-en-3-one; T0027; 17b-hydroxy-(8a,10a)-androst-4-en-3-one; 17b-hydroxy-(9b,10a)-androst-4-en-3-one; BIM-0061761.0001; C00535; D00075; rac-17b-hydroxy-(9b,10a)androst-4-en-3-one; S00309; AB00973630-03; Testosterone, VETRANAL(TM), analytical standard; Androst-4-en-3-one, 17-hydroxy, (17.beta.)-; Q-101251; Q1318776; B5DEE83F-632B-48A1-A0ED-A51E7F13DF2E; TESTOSTERONE ENANTATE IMPURITY D [EP IMPURITY]; TESTOSTERONE PROPIONATE IMPURITY C [EP IMPURITY]; Testosterone, European Pharmacopoeia (EP) Reference Standard; Testosterone; 4-Androsten-17?-ol-3-one; 17?-Hydroxy-4-androsten-3-one; Testosterone for impurity D identification, European Pharmacopoeia (EP) Reference Standard; Testosterone for system suitability, European Pharmacopoeia (EP) Reference Standard; (1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0;{2,7}.0;{11,15}]heptadec-6-en-5-one; 1050678-68-6; 17?-hydroxyandrost-4-en-3-one; epitestosterone; 17-epi-Testosterone; 17?-cis-Testosterone; 4-Androstene-17?-ol-3-one; Isotestosterone
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
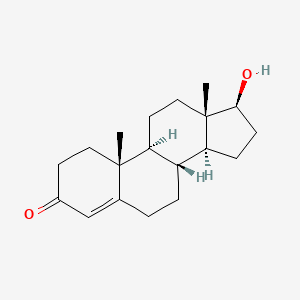 |
||||
| Formula |
C19H28O2
|
||||
| IUPAC Name |
(8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one
|
||||
| Canonical SMILES |
CC12CCC3C(C1CCC2O)CCC4=CC(=O)CCC34C
|
||||
| InChI |
InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1
|
||||
| InChIKey |
MUMGGOZAMZWBJJ-DYKIIFRCSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Prostate cancer | ICD-11: 2C82 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | LNCaP cells | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| LAPC-4 cells | Prostate carcinoma | Homo sapiens | CVCL_4744 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| NK-92 cells | Natural killer cell lymphoblastic leukemia | Homo sapiens | CVCL_2142 | ||
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | ||
| In Vivo Model |
Adult athymic nude mice were inoculated subcutaneously in the flank with the LNCaP human prostate cancer cell lines in 200 uL of Matrigel. Mice were divided into two groups, and the treatment group was implanted with 2 one cm long silastic implants filled with testosterone as described previously. Tumors were harvested 2- and 4-days post-treatment and fixed in 10% buffered formalin and processed for IHC and H&E staining.
Click to Show/Hide
|
||||
| Response regulation | Testosterone induces two parallel autophagy-mediated processes, ferritinophagy and nucleophagy, which then activate nucleic acid sensors to drive immune signaling pathways in prostate cancer. | ||||
